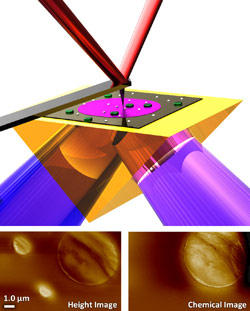
The sample (green/white) absorbs infrared laser light (purple) at wavelengths determined by its chemical composition, causing it to expand, which deflects the AFM cantilever. Bottom left: The AFM detects the height of two small polystyrene particles and a large polymethylmethacrylate (PMMA) particle. Bottom right: The light is tuned to be absorbed only by PMMA but not by polystyrene. Combining the data and recording chemical images at different wavelengths produces a map of the surface's topography and chemistry.
Researchers at the National Institute of Standards and Technology (NIST) and the University of Maryland have demonstrated that a new spectroscopy technique can simultaneously measure a material's topography and chemical composition with nanometer-scale spatial resolution.
While infrared (IR) microscopy has been used since the 1950s to determine the chemical composition of materials, the spatial resolution of the technique has been limited to tens of micrometers; the new approach reported by NIST overcomes this limitation, improving the spatial resolution by a factor of about a thousand while retaining the high chemical specificity of IR spectroscopy.
The technique, called photothermal induced resonance (PTIR), was demonstrated first at the University of Paris-Sud. It uses a tunable infrared laser and an atomic force microscope (AFM) to extract chemical information with nanometer-scale spatial resolution. Every material has a unique infrared spectrum that acts like a chemical fingerprint, and as the laser scans across its surface, the sample at each point absorbs IR light at wavelengths that are determined by the chemical composition at that spot. The absorbed light heats the material, causing it to expand ever so slightly, which can be detected by the AFM. Repeatedly scanning the sample at different wavelengths reveals the sample's underlying chemical composition with a resolution determined by the AFM tip size and the sample's thermo-mechanical properties, resulting in a resolution many times smaller than IR microscopy, which depends on the wavelength of the light. Since the properties of nanomaterials depend on their shape, size and chemical composition, the team's work gives researchers a powerful tool to measure some of the key characteristics of their creations.
For the first time, the NIST scientists have demonstrated that PTIR can be used to generate a signal that is proportional to the energy absorbed by a material, a necessary step for accurately measuring the relative amounts of different chemicals within. The scientists fabricated samples with features having different chemical compositions as narrow as 100 nm wide and as thin as 40 nm. Using PTIR, they were able to distinguish the different chemical components down to the smallest features, suggesting that even better resolution may be achievable.
"What's extraordinary is that we can see that the chemical map is not necessarily correlated to the height or size of the physical features on the sample surface," says Andrea Centrone, a scientist from the University of Maryland's Institute for Research in Electronics and Applied Physics working in NIST's Center for Nanoscale Science and Technology. "We get independent details of both the surface's physical features and its chemical properties. This result is unmatched by other near-field techniques."
According to Centrone, the results show that PTIR can dramatically exceed the resolution of IR microscopy. The research team is planning further experiments to extend the technique's capabilities and performance.

