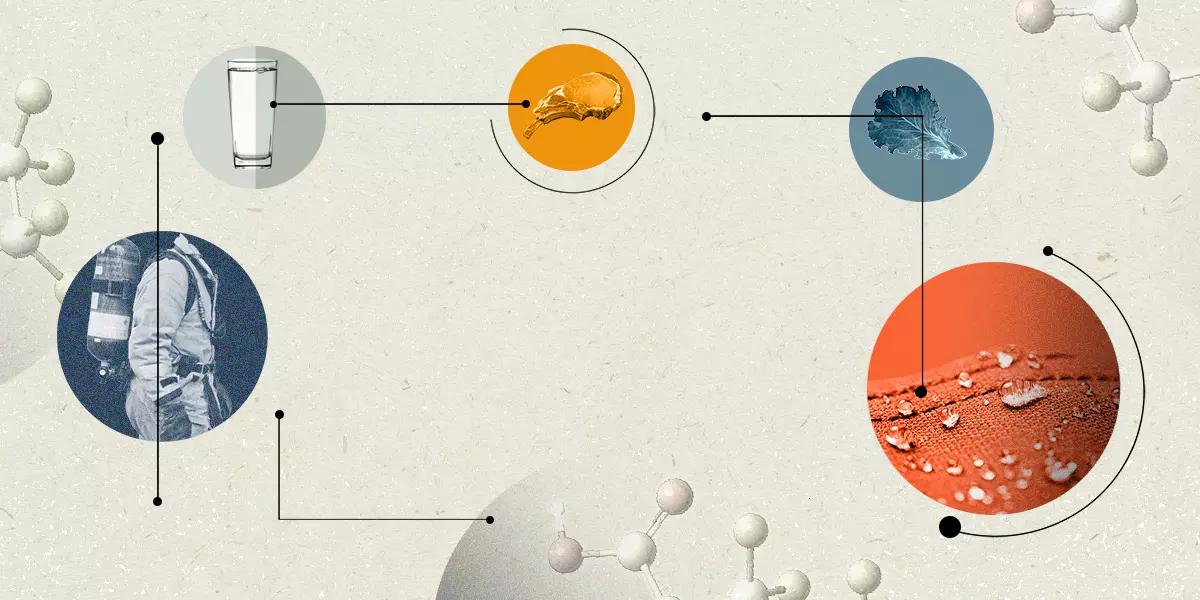
They’re in the air we breathe, the water we drink, the food we eat and the soil where that food was grown.
They’re in our carpets, our cookware, our drinking straws, our cosmetics and our clothes.
They’re in medical products, mining chemicals, clean-energy technology and the equipment that manufactures our computer chips.
Finding PFAS Wherever They’re Hiding
NIST scientists are helping reveal tiny amounts of ‘forever chemicals’ in our food, water, clothing and environment.
They’re called per- and polyfluoroalkyl substances, or PFAS, a group of thousands of compounds that contain a chemical bond between fluorine and carbon. That bond has proved to be one of the most stable and unbreakable known to chemistry — a fact baked into the common nickname “forever chemicals,” because once PFAS are created, they last a very long time.
First manufactured in the 1940s, PFAS have seeped into our daily lives, and our bodies. In recent years, they have emerged as a serious public health concern. Scientists have reported evidence that certain PFAS, at high enough concentrations, may harm health by suppressing the immune system or causing cancers, obesity, thyroid problems and birth defects.
Forever chemicals have also been found in remote forests, in the Arctic and at the bottom of the ocean. Reminiscent of pesticides like DDT in the 1960s and PCBs in the 1970s, they’ve emerged as some of the most pervasive and troublesome environmental contaminants of our time.
At the same time, health researchers are still determining how harmful PFAS are at the levels most of us are exposed to. And some of the studies that have raised the most alarms have used measurement methods that can overestimate the chemicals’ concentrations.
Researchers and regulators now face a daunting task: They must accurately measure PFAS in the countless places they’ve ended up and assess when and where these compounds have reached dangerous levels. To do so, scientists must often measure the chemicals at extremely low concentrations. PFAS can be found in food, drinking water and other materials in concentrations of parts per billion or even parts per trillion — equivalent to a few drops in an Olympic-size swimming pool.
This is where the National Institute of Standards and Technology (NIST) comes in. NIST scientists have pioneered methods that have made laboratory tests for PFAS in food, water, soil, firefighter gear and other materials more accurate. NIST research will support laboratory tests needed to implement the nation’s first-ever PFAS drinking water regulations, which the Environmental Protection Agency (EPA) released on April 10, 2024.
NIST researchers have also produced one of the world’s largest public databases of empirically measured PFAS masses, to help other researchers and labs more efficiently sniff out these troublesome compounds.
PFAS represent the kind of measurement challenge that NIST was made to tackle.
“In the federal government space, NIST was ahead of the game on PFAS,” says NIST research chemist Jessica Reiner.
A Useful but Troublesome Chemistry
The PFAS industry started in the late 1930s and early 1940s, after an engineer at the company DuPont invented a fluoropolymer that became known as PTFE. Marketed as Teflon, PTFE became a blockbuster, launching a multibillion-dollar industry of nonstick coatings applied to cookware and an almost unlimited range of other products.
Over the ensuing decades, thousands of PFAS were manufactured and incorporated into industrial lubricants, water-repellent clothing and carpeting, food packaging and more. If you’ve ever marveled how the wrapper around a greasy fast-food burger keeps your hands clean, or how effortlessly your lightweight rain jacket sheds water, you can probably thank a PFAS.
“Chemically they’re amazing — they do amazing things,” says John Kucklick, leader of the Biochemical and Exposure Science Group at NIST. “Anything that repels grease is probably fluorinated.”
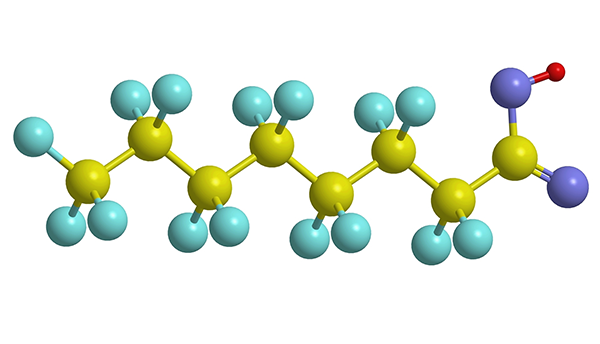
The chain of carbon atoms (yellow balls) and fluorine atoms (blue balls) characteristic of PFAS molecules is uniquely good at repelling water and grease.
Chemical companies had evidence of PFAS-related health impacts as early as the 1950s. But only in the late 1990s and early 2000s did government agencies such as the EPA take a hard look at the chemicals, after a lawsuit exposed DuPont-led studies of their harmful effects on factory workers. Around that time, researchers at Michigan State University found PFOS, a major PFAS of concern, in the tissues of fish, birds and mammals throughout Europe and North America, including the Arctic. “That really lit the fuse for all this PFAS research,” says Kucklick. “It became a very hot topic.”
PFAS had proliferated far faster than scientists were able to study each one in detail, creating an urgent need to accurately measure the compounds in all kinds of contexts. Among the first things NIST scientists did was test for PFAS in the tissues of animals kept in the institute’s biorepository, based in Charleston, South Carolina. The repository contains frozen tissue from marine mammals and sea turtles that were collected starting in the 1980s. NIST researchers led by biologist Jennifer Lynch found PFOS and other PFAS in sea turtle blood and marine mammal livers — a concerning result that was consistent with other published studies.
There were also more encouraging findings. Lynch’s liver measurements revealed that manufacturers’ voluntary phase-outs of certain PFAS starting in the early 2000s had, over time, reduced the levels accumulating in animals.
Reiner kick-started another PFAS-related research program when she joined NIST in 2008. She had studied with the author of the influential animal contamination study and done a postdoc at the EPA. But after a 2004 paper by an international group of researchers detailed how PFAS measurements could go awry — and suggested that NIST could provide standards — Reiner “realized we had a lot of measurement problems” to solve. At the time, different labs' estimates of PFAS concentrations in the same material deviated by as much as several hundred percent. This made the measurements hard to trust. “It was the wild west,” says Reiner.
In the federal government space, NIST was ahead of the game on PFAS.” —Jessica Reiner, NIST research chemist
One problem involved compounds that can mimic PFAS in a lab test, leading to errors. Eggs, for example, contain a bile acid that can easily be mistaken for certain PFAS. In a particularly high-profile case, when a scientist with the Food and Drug Administration presented a preliminary finding of high PFAS levels in chocolate cake, the report ignited a media firestorm. “That chocolate cake won’t last forever, but the chemicals in it might,” one headline stated. Follow-up testing by the FDA soon revealed the finding to be a false positive; the cake contained no detectable PFAS.
Building on Lynch’s research, Reiner first worked on measuring PFAS accurately in existing NIST reference materials, including human plasma, fish and house dust. These are well-characterized materials that any lab can purchase and measure with its own equipment. If the lab's measurement differs from the NIST-provided value on the label, its equipment needs to be adjusted or calibrated. NIST reference materials have helped make a wide range of important health and environment-related tests more accurate and reliable.
By 2012, Reiner’s team had produced new standard reference materials, or SRMs, for several of the PFAS most commonly found in biological samples. Over several years, the amount that different labs’ PFAS measurements deviated from the average plummeted from several hundred percent to around 40%, indicating that lab testing had become reasonably reliable. Eventually, Reiner thought, “OK, we’re good [with PFAS]; let’s move on.” She started working on flame retardants and plasticizers.
But events soon brought her back to PFAS. In 2016, New York State detected PFOA, another common PFAS, in the Village of Hoosick Falls’ public drinking water supply and Town of Hoosick private drinking water wells above the EPA health advisory level of 70 parts per trillion (ppt). PFOA, though no longer produced in the U.S., had been widely used to make products such as Teflon and foams for fighting fires. It has been linked to elevated cholesterol, thyroid disease, reduced immune response and some forms of cancer. Other studies soon found PFOA and other PFAS in drinking water systems around the country.
It turned out that Reiner’s — and NIST’s — work on PFAS was just beginning.
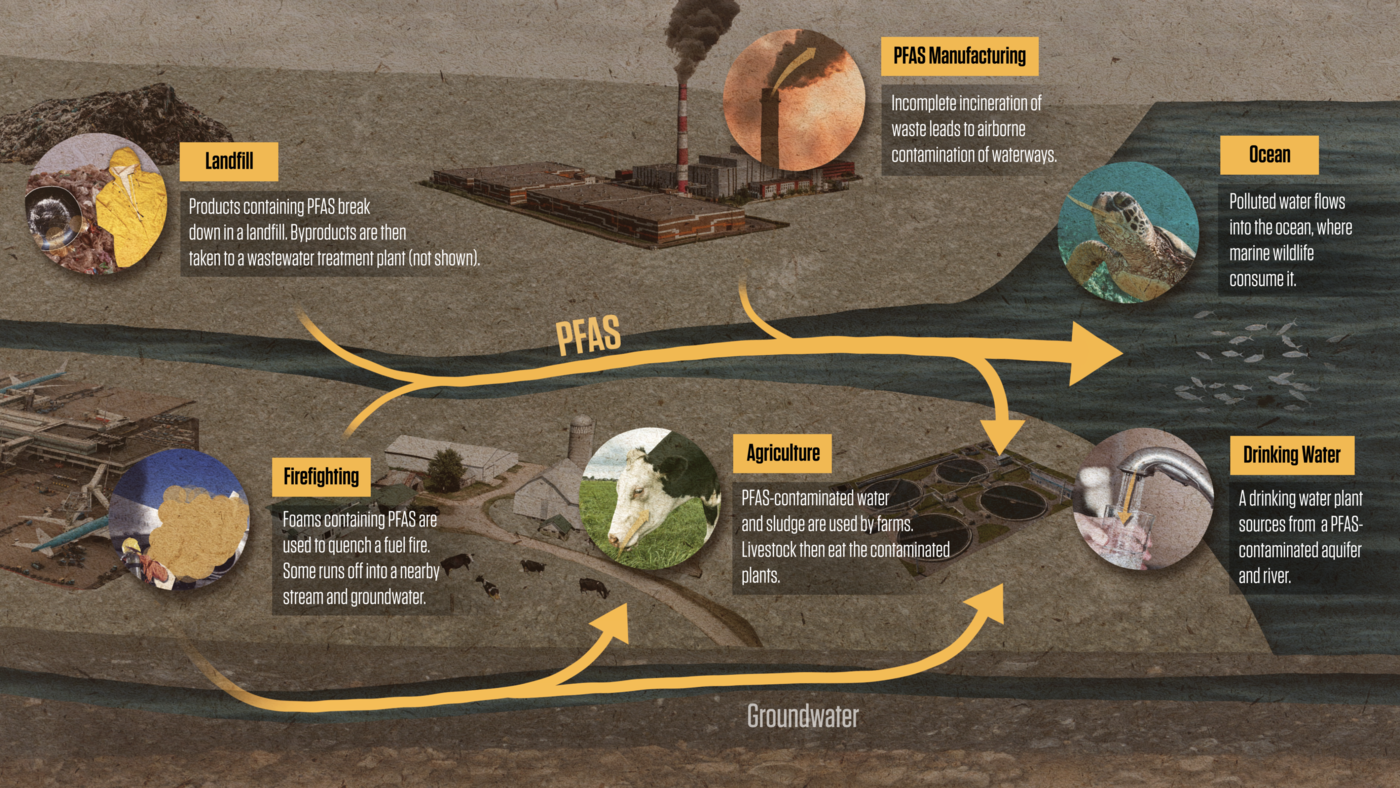
Because PFAS don’t break down, they have been able to move widely through the environment, contaminating everything from food to drinking water to marine wildlife.
It’s in the Water
While PFAS is everywhere, it’s made the largest public splash, so to speak, in water. Since the 2016 finding of PFOA in New York, PFAS that leaked or discharged from factories, military bases and other facilities have been found in 45% of the nation’s tap water, according to the U.S. Geological Survey. More than 20 states have since moved toward regulating the chemicals. In April 2024, the EPA announced the first national limits for six PFAS in drinking water. The agency set maximum concentrations of PFOA and PFOS, for example, at 4 parts per trillion.
To give regulated entities and broader society confidence, government and private labs will need to accurately quantify PFAS even at very low concentrations. That will require recognizing and controlling for issues that can mess up test results, says NIST research chemist Alix Rodowa. “PFAS are in so many things!” she says. “They’re in the instrumentation we use. Do we have Teflon tape on things? Is it in the materials we’re using to collect the samples? We have to think about all those things, especially at those ultra-trace levels.”

Alix Rodowa in the lab figuring out how to accurately measure PFAS in drinking water.
Rodowa and her colleagues have upgraded their measurement equipment with PFAS-free tubing and developed a suite of “tricks” to reduce contamination. She has been working on an SRM that labs can use to ensure their drinking water tests are accurate. Initially she was considering sending labs bottled water with a known amount of PFAS. But she realized the chemicals were sticking to the sides of the bottle, potentially compromising the analysis results.
So she switched to a two-pronged approach: ship out a standardized volume of tap water purified using reverse osmosis — among the most effective PFAS filtration methods available — along with an ampule of PFAS diluted in methanol to a known concentration. Methanol reduces intermolecular forces that cause the PFAS to stick to the bottle, allowing for a more uniform mixture of PFAS and water. Lab workers who purchase the SRM can pour the solution into the purified water and test it on their equipment.
The next step is to send the samples to labs to get feedback, which will help Rodowa further refine the material. Only when labs can consistently use the product to improve test results will NIST add it to its catalog of reference materials. The final product is still at least a year away — "SRMs are really hard to make,” Rodowa says — but when it comes out, “it will absolutely help. It will make the results much more comparable over time and build confidence for consumers of the commercial lab reports.”
The work could especially aid new labs coming online to meet the enormous and growing need for water testing, adds Reiner. “If they have a material like this to help them develop their method and get it set up correctly, they’re going to end up saving a lot of money and a lot of time.”
Fluorinated Food Fears
Few things are more frightening than the thought that the food you eat or feed to your family could contain harmful chemicals. And even if the FDA’s initial chocolate cake report proved a false alarm, PFAS has been showing up in food with distressing regularity.
NIST’s food-related PFAS work began with Reiner’s studies of fish from the Great Lakes that the institute had already used to make reference materials for other chemical contaminants. But the urgent need for additional PFAS-specific materials became clear a few years ago, when a dairy farmer in Maine discovered the chemicals in high concentrations in his cows, likely because they drank contaminated groundwater or ate contaminated feed. The farmer ended up making the heartbreaking decision to cull his herd. But there was a silver lining — through connections at the FDA and Maine’s public health department, NIST chemists Benjamin Place and Melissa Phillips bought around 135 kilograms (300 pounds) of meat from the farmer.
The meat arrived in the form of frozen patties. But figuring out how to accurately measure concentrations of PFAS in such a complicated material was far from trivial. The patties were first sent to the Hollings Marine Lab in Charleston, South Carolina, where they were chilled to extremely low temperatures and milled into a powder. Then, Place and his colleagues had to solve various challenges, like preventing the pinkish-red, slurried meat from sticking to lab equipment and test tubes. The process has proved to be one of the more, shall we say, visceral ones NIST researchers have encountered.

NIST’s Melissa Phillips holds a bag of frozen spinach, while Ben Place holds a vial of frozen ground meat. Both are destined for future SRMs that will help labs accurately measure PFAS in food.
Place and Rodowa are now working to create SRMs for cow and pig meat. Rodowa and Place have also started preparing an SRM based on PFAS-contaminated spinach. And they have plans in the works for animal feed based on fermented corn stalks, which are currently stored in a NIST freezer.
With more and more states discovering PFAS-contaminated food and developing regulations around the chemicals, demand for these products is likely to be high. Already, NIST’s 15 PFAS-related SRMs have become some of the agency’s most popular products. Sales of the Great Lakes fish SRMs doubled between 2019 and 2021; the materials sometimes sell out. “They’ve been flying off the shelves,” says NIST research chemist Kate Rimmer.
Firefighters in the Spotlight
Chemicals in water and food affect everyone. But certain groups are far more likely than the rest of us to be exposed to high levels of PFAS.
One of those groups includes the people who protect us and our homes from fire. Firefighters have raised alarms as studies have found elevated PFAS levels in their blood compared with the general population, along with higher rates of certain cancers.
While firefighters could be exposed to PFAS from multiple sources, turnout gear — the familiar yellow and tan outfits firefighters don before plunging into a burning building — has become a prime suspect, thanks in part to university studies that have garnered media attention. This gear must meet stringent requirements for flame retardance and water repellence. And one of the most reliable ways for manufacturers to meet the performance standards is to use PFAS.

A firefighter’s protective "turnout gear" is composed of three distinct layers made of different textiles. In response to concerns about the gear possibly exposing firefighters to PFAS — several of which have been linked to cancer — NIST researchers investigated the presence of the chemicals in textiles used to make the layers.
In 2020, a bill sponsored by Sen. Jeanne Shaheen of New Hampshire directed NIST to identify and quantify the PFAS in turnout gear.
The congressional funding enabled the research team to buy dozens of PFAS standards — commercially available solutions with precisely calibrated concentrations of the PFAS of interest — to calibrate its equipment. Each standard can run thousands of dollars. The researchers ultimately analyzed 53 compounds and 20 pieces of brand-new turnout gear — the most comprehensive examination published to date.
Due to the high cost of buying all the gear and chemical standards and the amount of work involved, “I do not think this study could have been done by anybody else other than the federal government,” says Rick Davis, the NIST materials research engineer who led the research.
The team’s first report, released in May 2023, bore both good and troubling news. On the concerning side, all the fabrics contained some PFAS. The moisture barriers — the middle layer firefighters wear — and the outer shells the researchers tested contained the highest concentrations of the chemicals, up to around one part per million. Davis says the chemicals are likely added to make turnout gear waterproof, and that gear manufacturers may be able to achieve similar performance levels without using PFAS.
I do not think this study could have been done by anybody else other than the federal government.” —Rick Davis, NIST materials research engineer
More positively, the thermal liners firefighters wear against their skin contained much less PFAS. Moreover, the chemicals found in high concentrations in the other layers were not PFOA and PFOS — the so-called long-chain PFAS that have been most intensively studied but also largely phased out — but rather chemicals with shorter carbon chains. Such short-chain PFAS are more quickly flushed from the body, though it’s not clear whether that makes them safer.
A second study released in January 2024 examined gear that had been heated, abraded or weathered — the kinds of wear expected when battling a fire. The team found that abrasion caused the greatest increase of PFAS in all fabric types, whereas heating increased PFAS concentrations substantially in the outer shells and slightly in the thermal liners, while decreasing them in the moisture barriers. Weathered outer shells also showed a large increase in PFAS.
On the other hand, when gear was laundered, PFAS concentrations declined slightly — though the released chemicals probably ended up in the wastewater stream, raising potential concerns about environmental contamination.
Davis stresses that his team’s results do not address whether firefighters’ gear is harming them. “These studies better define the PFAS a firefighter could be exposed to from their gear,” he says, “which gives a narrower PFAS focus for those who will conduct firefighter health exposure studies.” Researchers at universities and other agencies are just starting to examine the health impacts of short-chain PFAS; one major unknown is how readily they move from clothing into the wearer’s body. The NIST studies aim to provide a rigorous and unbiased foundation for future decisions around firefighting gear.
And the work is far from done. Davis’s next project will look at PFAS in firefighter gloves, hoods and other gear. Another future study will examine PFAS at fire stations and other workplace exposures.
Another way firefighters could be exposed to PFAS is through aqueous firefighting foams. Starting in the 1960s, these products were used to quench flaming liquids like gasoline at sites such as military bases, oil refineries, airports and chemical factories. PFAS-based surfactants in these foams, which have proved uniquely good at smothering intense, high-temperature fires, can constitute up to 6% of the product’s total weight — an extremely high concentration found virtually nowhere else. During military training exercises, the chemicals often ended up in concrete pits and, from there, leaked into nearby groundwater. In some cases, water contaminated a half-century ago still appears foamy.
Congress has ordered the Department of Defense — the top user of these foams — to phase out most PFAS-containing firefighting foams by 2024. But legacy foams that remain on bases and other facilities could continue to contaminate the environment. To help ensure the fire suppressants people are using are PFAS-free, Reiner and other NIST researchers worked to develop SRMs for foams. The project was challenging, Reiner says, because the foam created when these products are used turned out to have a higher PFAS concentration than the overall product, so if a lab measures just the foam, it will get too high a value.
In September, the team announced four new SRMs with formulations of PFAS found in several major types of firefighting foams. Labs around the country can use these materials to calibrate the equipment they use to analyze foams for PFAS, Reiner says.

A series of reference materials contain precise measurements of per- and polyfluoroalkyl substances (PFAS), known as forever chemicals, in firefighting foams. These foams, called aqueous film-forming foams (AFFFs), are used to suppress fuel fires. Analytical labs can use the reference materials for measuring PFAS in the foams so they can be removed.
A Measurement and Mitigation Challenge
Like them or not, PFAS are part of our world — and not all are necessarily acutely dangerous. Fluorinated polymers used to lubricate surfaces and prevent abrasion, for example, are relatively stable and seem to present less urgent health or environmental risks than more mobile compounds like PFOA and PFOS. Such polymers appear in manufacturing equipment used for semiconductors and many clean-energy technologies, highlighting their importance to the modern economy. A push to purge them all would be costly.
And in some cases, it may be that only PFAS can do the job. If gasoline ignites on a ship or in an airport, you probably want whatever will quench the flames most quickly, even if it contains PFAS.
On the other hand, some products that currently contain PFAS, such as carpets and food wrappers, may not need them. (Indeed, the FDA recently announced that food packaging containing PFAS would no longer be sold in the U.S.) And reducing levels of the chemicals in food, water and the environment is a clear priority.
NIST’s forthcoming drinking water SRM “will make the results much more comparable over time and build confidence for consumers of the commercial lab reports.” —Alix Rodowa, NIST research chemist
The challenge facing measurement scientists is to pinpoint the concentrations and situations in which PFAS is truly harmful — and to communicate that science effectively, so that the public is empowered and not just alarmed. The proliferation of PFAS — nearly 15,000 have been identified, according to the EPA — has made this an especially daunting challenge.
One way NIST is helping is by creating a database of PFAS chemical structures and masses. Researchers with a sample they suspect might contain PFAS can run a process called mass spectrometry and check their results against the NIST database to look for a match. The database contains 132 structures, including many of the most commonly found PFAS compounds and fragments, making it one of the largest public databases of PFAS masses. And there are plans to add more, including by allowing outside researchers to add their own data.
“We’re hoping that if we can do the hard part and identify those chemicals, others won’t have to,” says Jared Ragland, a NIST research biologist who is leading the database effort.
A second, newly created NIST-based list includes compounds such as the bile acid that can confound PFAS measurements. Within days of a paper about the dataset coming out, Rodowa says she had already received information about four additional PFAS-mimicking substances, highlighting the need for such a resource. She and Reiner, who developed the list along with colleagues at the EPA, FDA and several other institutions, hope it will help prevent reports of exaggerated PFAS concentrations or false positives — which Reiner says can sometimes cause more harm than the chemicals themselves.
By helping the world accurately identify and measure PFAS, NIST scientists aim to guide us toward a safer, cleaner and, hopefully, more empowered and less fearful future. “We need to give people the right information,” Reiner says, “so they can make more informed decisions.”
Editor’s Note: This story was updated to include information on the national limits for PFAS in water announced by the Environmental Protection Agency on April 10, 2024.
Learn More About NIST’s Work Tackling PFAS Measurement Challenges
Spotlight: Alix Rodowa and PFAS in Groundwater
Creating a PFAS-Contaminated Meat SRM: A Q&A With NIST Chemists Melissa Phillips and Ben Place
Addressing Measurement Challenges for Detecting Chemicals That Could Cause Cancer
New NIST Database of ‘Forever Chemicals’ Will Help Scientists Monitor Environmental Pollution

