Summary
The growing sophistication of electronic medical devices results in two broad benefits for both individuals and society: improved survivability in the face of disease or injury and greatly enhanced quality of life. However, for both economic and public acceptance reasons, these benefits require that the medical devices maintain high reliability over well-defined lifetimes. To assist the medical electronics industry in achieving the needed reliability, this project is intended to provide critical test data, standard test structures and standard test procedures to provide understanding of failure processes, thereby improving reliability of the devices.
Description
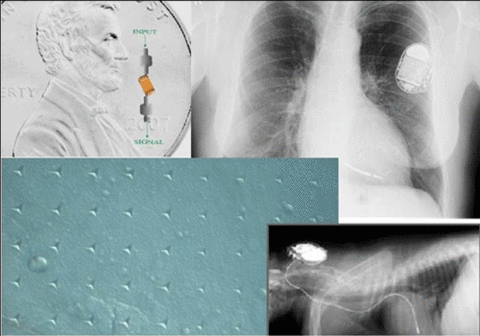
Impact and Customers
This project will reduce the need for surgeries to replace failed implants, thereby improving safety and quality of life for implant recipients and reducing medical expense to society. It will provide assessment tools for the external electronic devices that support the implants, thereby decreasing time to market and regulatory barriers.
Capacitor and board fabrication companies have indicated that they would use nondestructive evaluation inspection tools, if available.
Knowledge of failure processes and development of reliability protocols for parylene C will provide companies and the Food and Drug Administration (FDA) with tools for assessing proposed coated implanted electronic devices.
Three industry-driven projects—medical electronic components, implanted electronic leads, and portable electronic medical devices—will result in industry-accepted reliability criteria.
Customers include
- International Electronics Manufacturing Initiative
- Food and Drug Adminisration
- Industry partners
Approach

Capacitor nondestructive evaluation and parylene C activities involve distinct approaches, although certain tools are common to both: optical and electron microscopies, furnace thermal treatments, post-measurement sectioning and failure analysis.
Capacitor nondestructive evaluation work focuses on ultrasonic resonance measurements because structural damage changes the resonance spectra of the capacitors. Isolated capacitors are measured in a two-point, continuous wave, ultrasonic transducer-driven instrument from 1 MHz – 3.5 MHz. Mounted capacitors are tested over the same frequency range but are driven by the ferroelectric properties of the capacitors themselves; i.e., a DC voltage is applied to the capacitors to align domains and an AC voltage is simultaneously applied to activate the resonance vibrations. Nonlinear properties are investigated by applying pulsed voltages to the capacitors and monitoring the resultant signal decay and Fourier transform.
Parylene properties are measured by a frost-point water permeation system, a nanoindentor for measuring elastic, viscoelastic, and hardness properties, and a profilometer to measure substrate and coating surface geometries. Colleagues at the FDA are providing X-ray spectroscopy measurements.
Major Accomplishments
We have shown that, while large-scale accelerated testing will detect defective batches of capacitors, e.g., caused by contaminated dielectric, it will not eliminate the short lifetime capacitors that result in premature failure and necessitate early, expensive and potentially life-threatening surgery. Those capacitors that result in early device failure were found to be independent of all accelerated failure test parameters and of manufacturer. It was concluded that early device failure was caused by isolated capacitors damaged either during fabrication or during mounting. Nondestructive evaluation protocols that can provide 100% inspection for both free-standing and mounted capacitors are required to eliminate these components. Such nondestructive evaluation tools are being developed for both isolated and mounted capacitors, based upon changes in ultrasonic resonance spectra. We have demonstrated that as-received free-standing and mounted capacitors exhibit the same resonance frequencies (±25 KHz), but capacitors that show unusual leakage current behavior after accelerated testing have quite different resonance spectra. This suggests that ultrasonic resonance spectra may provide a viable tool for detecting damage in capacitors at levels well below those required to modify actual capacitance values.
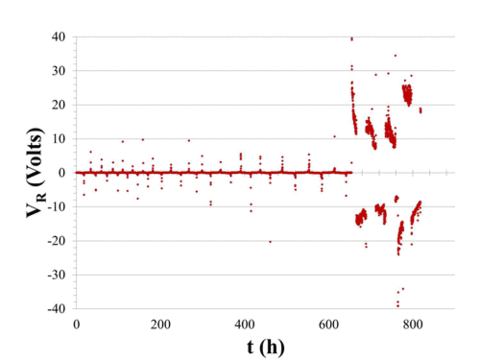
|
Figure 1. Leakage current (VR/106 A) showing capacitor failure about 650 h into test. |
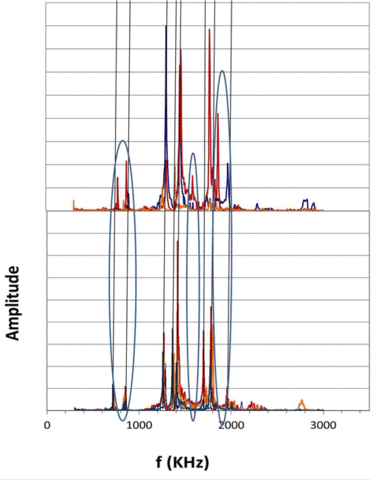
|
Figure 2. Resonant ultrasound spectra of 4 as-received (bottom) and damaged (top) showing shifts in spectra resulting from accelerated testing. |
Mechanical properties of parylene C, currently used to coat implants such as stents and proposed for coating implantable electronic devices, have been shown to depend upon the chemistry of substrates even for thick (e.g., 16 µm) coatings. Parylene coatings laid down upon Al, Cu, and stainless steel substrates had 10% variation in indentation elastic constant and hardness values, although both elastic constant and hardness were highly reproducible for a given substrate. The error bars in Figure 3 represent the 95% confidence range. This variation does not correlate with the elastic properties of the substrates, indicating that there is a chemical effect of the substrates on the mechanical properties of the coatings even for 16 µm thicknesses.
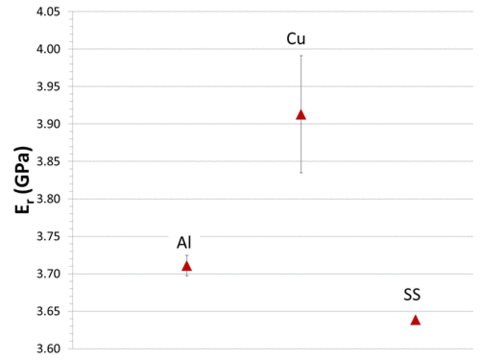
|
Figure 3. Elastic constant (reduced modulus) at surface of 16 µm thick parylene C coating for three substrates, Al, Cu and stainless steel, based upon 300 measurements for each substrate. |
Steady state water permeating through multiple 16 µm thick free-standing parylene coatings has been measured to be 3 ppm to 5 ppm at our measurement gas flow rate. Heating one of the coatings to 120 oC increased the steady state water concentration to 25 ppm, suggesting that permeation is strongly influenced by the crystal to amorphous ratio in the coatings.
An industry-driven consortia addressing the high failure rates of implanted electric leads has just requested that NIST assess the individual companies' proprietary lead testing procedures and develop a "best practice" test procedure for the industry.
Two activities from the International Electronics Manufacturing Initiative in which project members participate, Component Specifications for Medical Products and Qualifications for Portable Medical Products, have just been converted to official projects by the initiative. The goal of the projects is development of industry-adopted reliability acceptance criteria for medical-grade electronic components and portable devices.
Project brief (PDF)

