Who cares about old plastic? Researchers at the National Institute of Standards and Technology (NIST) do, so that you won't have to years down the road, when today's plastic concoctions start to break down and disintegrate from weather exposure. Experiments at NIST may help scientists devise better tests to make sure aging plastics won't turn into environmental or health hazards as time goes by.
Tests like this are more important now than ever, because plastics aren't what they used to be. Modern epoxies are frequently made stronger, lighter and more resilient with the addition of multiwalled carbon nanotubes (MWCNTs), a special form of carbon that under a microscope looks like rolls of chicken wire. MWCNTs already enhance plastics used in baseball bats, tennis rackets, bikes and airplanes, and though the tiny tubes appear to be long-lasting, no comprehensive set of tests exists to determine what happens to them over the long haul. So a NIST team took steps to change that.
"Some studies have been done about the effect of ultraviolet (UV) light, but not with a large number of analytical methods," says NIST's Elijah Petersen. "We wanted to begin developing a suite of tests for evaluating the performance of these nanocomposite materials, so that we can examine their potential risks, if any, during usage."
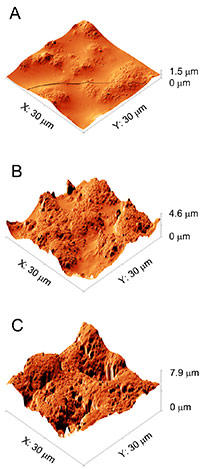
The team needed a way to simulate the degrading effect of years of high temperature, humidity and sunlight, but without waiting that long for results. They created samples of epoxy with 3.5 percent MWCNTs—a fairly typical mixture quantity—and put them into a NIST-developed device called SPHERE (Simulated Photodegradation via High Energy Radiant Exposure), which pours out powerful UV light into a chamber kept at 50 degrees Celsius and 75 percent humidity. Keeping the samples there for 100 days "was the equivalent of four years in the Florida sun," Petersen says.
After exposing the samples to this artificial Florida, the team ran a set of six different tests that analyzed changes ranging from mass to appearance to surface chemistry. One major discovery was that the UV light tended to destroy the epoxy, but on the surface remained a spaghetti-like network of nanotubes, which the group of tests indicated were less damaged by SPHERE exposure than the epoxy matrix.
So are these nanocomposite materials stable forevermore? Petersen says the study did not answer every question, but that it should help researchers determine answers more effectively than was possible before by development and optimization of multiple analytical methods.
"We got a lot of new information from the test suite about how nanocomposites degrade," says Petersen, "and the most encouraging thing is that the results of the different tests generally back one another up. We hope the test suite allows for better analysis of the stability of these new plastics, which are growing more common in everyday life."
E.J. Petersen, T. Lam, J.M. Gorham, K.C Scott, C.J. Long, D. Stanley, R. Sharma, J.A. Liddle, B. Pellegrin and T. Nguyen. Impact of UV irradiation on the surface Chemistry and structure of multiwall carbon nanotube epoxy nanocomposites. Carbon, Dec. 16, 2013, doi: 10.1016/j.carbon.2013.12.016.

