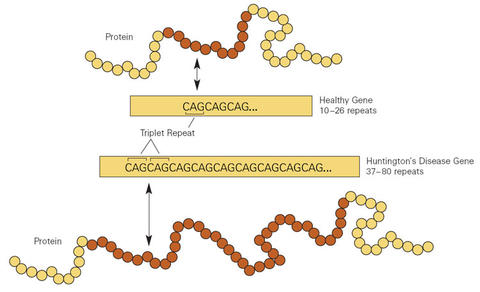
Graphic showing the excessive repetitions of the cytosine-adenine-guanine (CAG) nucleotide sequence in a gene from a Huntington's disease patient (bottom) compared to a gene from a person without the neurodegenerative disorder (top).
A new Standard Reference Material (SRM) from the National Institute of Standards and Technology (NIST) will help clinical genetics labs accurately diagnose Huntington's disease, an inherited degenerative brain disorder that usually begins between ages 35 and 50 and progressively leads to physical impairment, dementia and death. A person whose mother or father developed Huntington's disease has a 50-50 chance of getting the currently incurable disease.
Huntington's disease results from a genetic mutation affecting approximately one in 10,000 persons. The mutation is characterized by an excessive number of repeats of a sequence of three nucleotides (the chemical building blocks of DNA), cytosine-adenine-guanine (CAG), located on the fourth of the 23 pairs of chromosomes found in every human cell. Since 1993, a genetic test has been available to count the number of CAG "triplet repeats" that exist, determining if a person will develop Huntington's disease, and if so, how severe it will be.
Individuals with up to 26 repeats are normal. Individuals with 27 to 35 repeats also are unaffected, but the number of repeats can increase in their children. Individuals with 36 to 39 repeats may or may not develop symptoms of Huntington's disease; however, if they do, it will likely be at a much later onset and slower progression than more pronounced cases. Individuals with 40 or more repeats will definitely be affected, while individuals with 60 or more repeats will develop symptoms in childhood.
Electing to be tested for Huntington's disease is an extremely difficult choice. Since a positive diagnosis undoubtedly will affect decisions about careers, relationships, having children and other life events, there is no room for error. But errors can occur sometimes because the test requires making many copies of the patient's DNA using the polymerase chain reaction (PCR), the standard technique for "amplifying" or making multiple copies of a DNA molecule. On rare occasions, PCR creates extraneous CAG repeats—an anomaly known as "stutter"—that make the triplet count appear higher than it really is.
Measures of the overall length of the DNA molecule—and in turn, the CAG repeat count—are greatly improved when the new NIST reference, SRM 2393, "CAG Repeat Length Mutation in Huntington's Disease," is used as a quality control. The SRM consists of six samples of DNA measured and certified by NIST for triplet repeats ranging in number from 15 to 75. The certified values are free of stutter, providing genetic screeners a more viable standard with which to compare a patient's DNA sample.
SRM 2393 joins more than 50 reference materials produced by NIST for quality control in clinical testing. It also is the latest response by NIST to the call from the health care community for higher-order reference materials for genetic diagnostic tests (the last being an SRM to improve the accuracy of Fragile X syndrome diagnoses). Standard Reference Materials are among the most widely distributed and used products from NIST. The agency prepares, analyzes and distributes about 1,300 different materials that are used throughout the world to check the accuracy of instruments, validate test procedures and serve as the basis for quality control standards worldwide.
To get information on purchasing SRM 2393 and download the certificate, go to https://www-s.nist.gov/srmors/view_detail.cfm?srm=2393.

