Summary
The National Institute of Standards and Technology (NIST) and the National Institutes of Health (NIH) Office of Dietary Supplements (ODS) established a Vitamin D Metabolites Quality Assurance Program (VitDQAP) in 2009. The VitDQAP conducted 12 exercises before concluding in 2015. Parts of the VitDQAP community were served through the HAMQAP from 2017 to 2021, and are now served through the ClinQAP.
Description
SRM 972a Vitamin D Metabolites in Frozen Human Serum
Interlaboratory comparison studies of the VitDQAP supported the reliability of vitamin D measurements, including determination of 25-hydroxyvitamin D2, 25-hydroxyvitamin D3, and 3- epi-25-hydroxyvitamin D3 in human serum and plasma. Results from the comparison studies helped participants to make accurate clinical and health-care decisions as well as to maintain and improve their measurement comparability.
Major Accomplishments
The VitDQAP administered 12 interlaboratory comparison exercises for the measurement of vitamin D metabolites in human serum and plasma. The comparability of laboratory measurements for target analytes improved substantially over time through the development and promulgation of robust measurement technologies, identification and production of suitable reference materials, isolation and identification of measurement system biases, and support and encouragement of within-laboratory measurement quality control efforts. The major accomplishments of VitDQAP have been summarized in a final report.
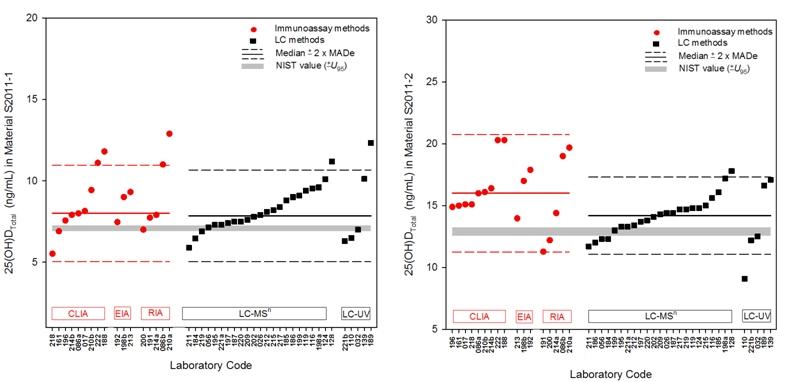
An example figure from the Summer 2011 Report illustrates the 25-hydroxyvitamin D (25(OH)DTotal) levels in two study materials as determined by immunoassay (CLIA, EIA, and RIA) and LC (LC-MSn and LC-UV) methods.
STUDY REPORTS AND WORKSHOPS
Reports for intercomparisons are available as NIST Interagency/Internal Reports (IRs):
| Exercise | Date | NIST IR |
|---|---|---|
| 1 | Winter 2010 | 7890 |
| 2 | Summer 2010 | 7891 |
| 3 | Winter 2011 | 7892 |
| 4 | Summer 2011 | 7893 |
| 5 | Winter 2012 | 7894 |
| 6 | Summer 2012 | 7895 |
| 7 | Winter 2013 | 8000 |
| 8 | Summer 2013 | 8133 |
| 9 | Winter 2014 | 8141 |
| 10 | Summer 2014 | 8142 |
| 11 | Winter 2015 | 8143 |
| 12 | Summer 2015 | 8169 |
Certificates of participation are also available upon individual request
VitDQAP workshops (or webinars) were held periodically, providing an opportunity for participants to discuss analytical methods, results of the interlaboratory comparison exercises, and other related topics.
RELATED STANDARD REFERENCE MATERIALS
NIST has developed a number of Standard Reference Materials (SRMs®) that are intended for use in validating methods for determining vitamin D metabolites in human serum and qualifying control materials produced in-house and analyzed using those methods.
These SRMs may be purchased from the Office of Reference Materials at NIST.
- SRM 972a Vitamin D Metabolites in Human Serum
- SRM 2969 Vitamin D Metabolites in Frozen Human Serum (Total 25-Hydroxyvitamin D Low Level)
- SRM 2970 Vitamin D Metabolites in Frozen Human Serum (25-Hydroxyvitamin D2 High Level)
- SRM 2972b 25-Hydroxyvitamin D Calibration Solutions
- SRM 2973 Vitamin D Metabolites in Frozen Human Serum (High Level)
Additional Technical Details
Frozen or freeze-dried serum or plasma samples together with control materials were sent to laboratories biannually (winter and summer) for analysis. Results were then returned to NIST for data evaluation and tabulation. Value-assignment of the samples was based on the median of all the laboratory results, with confirmation based on measurement at NIST using one or more different methods. Consultation and troubleshooting regarding methods of analysis were also provided. A report of exercise results was sent to participants, and workshops were scheduled periodically to discuss results as well as methodological issues and advancements in clinically relevant vitamin D metabolite measurements.
ASSOCIATED PUBLICATIONS
- Sempos, C. T., Williams, E. L., Carter, G. D., Jones, J., Camara, J. E., Burdette, C. Q., Hahm, G., Nalin, F., Duewer, D. L., Kuszak, A. J., Merkel, J., Hoofnagle, A. N., Lukas, P., Cavalier, E., Durazo-Arvizu, R. A., Crump, P. M., Popp, C., Beckert, C., Schultess, J., Van Slooten, G., Tourneur, C., Pease, C., Kaul, R., Villarreal, A., Ivison, F., Fischer, R., van den Ouweland, J. M. W., Ho, C. S., Law, E. W. K., Simard, J. N., Gonthier, R., Holmquist, B., Batista, M. C., Meadows, S., Cox, L., Jansen, E., Khan, D. A., Robyak, K., Creer, M. H., Kilbane, M., Twomey, P. J., Freeman, J., Parker, N., Yuan, J. Y., Fitzgerald, R., Mushtaq, S., Clarke, M. W., Breen, N., Simpson, C., and Wise, S. A., "Assessment of serum total 25-hydroxyvitamin D assays for Vitamin D External Quality Assessment Scheme (DEQAS) materials distributed at ambient and frozen conditions," Analytical and Bioanalytical Chemistry, 414, 1015-1028 (2022).
- Boggs, A. S. P., Kilpatrick, L. E., Burdette, C. Q., Tevis, D. S., Fultz, Z. A., Nelson, M. A., Jarrett, J. M., Kemp, J. V., Singh, R. J., Grebe, S. K. G., Wise, S. A., Kassim, B. L., and Long, S. E., "Development of a pregnancy-specific reference material for thyroid biomarkers, vitamin D, and nutritional trace elements in serum," Clinical Chemistry and Laboratory Medicine, 59, 671-679 (2021).
- Camara, J. E., Wise, S. A., Hoofnagle, A. N., Williams, E. L., Carter, G. D., Jones, J., Burdette, C. Q., Hahm, G., Nalin, F., Kuszak, A. J., Merkel, J., Durazo-Arvizu, R. A., Lukas, P., Cavalier, E., Popp, C., Beckert, C., Schultess, J., Van Slooten, G., Tourneur, C., Pease, C., Kaul, R., Villarreal, A., Ivison, F., Fischer, R., van den Ouweland, J. M. W., Ho, C. S., Law, E. W. K., Simard, J. N., Gonthier, R., Holmquist, B., Batista, M. C., Pham, H., Bennett, A., Meadows, S., Cox, L., Jansen, E., Khan, D. A., Robyak, K., Creer, M. H., Kilbane, M., Twomey, P. J., Freeman, J., Parker, N., Yuan, J. Y., Fitzgerald, R., Mushtaq, S., Clarke, M. W., Breen, N., Simpson, C., and Sempos, C. T., "Assessment of serum total 25-hydroxyvitamin D assay commutability of Standard Reference Materials and College of American Pathologists Accuracy-Based Vitamin D (ABVD) Scheme and Vitamin D External Quality Assessment Scheme (DEQAS) materials: Vitamin D Standardization Program (VDSP) Commutability Study 2," Analytical and Bioanalytical Chemistry, 413, 5067-5084 (2021).
- Burdette, C. Q., Camara, J. E., Nalin, F., Pritchett, J., Sander, L. C., Arid, G. D. C., Jones, J., Betz, J. M., Sempos, C. T., and Wise, S. A., "Establishing an Accuracy Basis for the Vitamin D External Quality Assessment Scheme (DEQAS)," Journal of Aoac International, 100, 1277-1287 (2017).
- Phinney, K. W., Sempos, C. T., Tai, S. S. C., Camara, J. E., Wise, S. A., Eckfeldt, J. H., Hoofnagle, A. N., Carter, G. D., Jones, J., Myers, G. L., Durazo-Arvizu, R., Miller, W. G., Bachmann, L. M., Young, I. S., Pettit, J., Caldwell, G., Liu, A., Brooks, S. P. J., Sarafin, K., Thamm, M., Mensink, G. S. M., Busch, M., Rabenberg, M., Cashman, K. D., Kiely, M., Galvin, K., Zhang, J. Y., Kinsella, M., Oh, K., Lee, S. W., Jung, C. L., Cox, L., Goldberg, G., Guberg, K., Meadows, S., and Prentice, A., "Baseline Assessment of 25-Hydroxyvitamin B Reference Material and Proficiency Testing/External Quality Assurance Material Commutability: A Vitamin B Standardization Program Study," Journal of Aoac International, 100, 1288-1293 (2017).
- Sempos, C. T., Betz, J. M., Camara, J. E., Carter, G. D., Cavalier, E., Clarke, M. W., Dowling, K. G., Durazo-Arvizu, R. A., Hoofnagle, A. N., Liu, A., Phinney, K. W., Sarafin, K., Wise, S. A., and Coates, P. M., "General Steps to Standardize the Laboratory Measurement of Serum Total 25-Hydroxyvitamin D," Journal of Aoac International, 100, 1230-1233 (2017).
- Wise, S. A., Tai, S. S. C., Nelson, M. A., Burdette, C. Q., Camara, J. E., Hoofnagle, A. N., Laha, T. J., Carter, G. D., Jones, J., Williams, E. L., Barclay, Z. J., Jones, G., Kaufmann, M., Binkley, N., Kapoor, A., Ziegler, T., Cashman, K. D., Dowling, K. G., and Sempos, C. T., "Interlaboratory Comparison for the Determination of 24,25-Dihydroxyvitamin D-3 in Human Serum Using Liquid Chromatography with Tandem Mass Spectrometry," Journal of Aoac International, 100, 1308-1317 (2017).
- Wise, S.A., Tai, S.S.-C. , Burdette, C.Q., Camara, J.E., Bedner, M., Lippa, K.A., Nelson, M.A., Nalin, F., Phinney, K.W., Sander, L.C., Betz, J.M., Sempos, C.T., Coates, P.M., (2017) Role of the National Institute of Standards and Technology (NIST) in Support of the Vitamin D Initiative of the National Institutes of Health, Office of Dietary Supplements, J. AOAC Int., 100(5), 1260-1276.
- Sander, L.C., Bedner, M., Duewer, D.L., Lippa, K.A., Phillips, M.M., Phinney, K.W., Rimmer, C.A., Schantz, M.M., Sharpless, K.E., Tai, S.S.-C., Brown, J.M., Wise, S.A., Wood, L.J., Betz, J.M., Coates, P.M. (2013), The Development and Implementation of Quality Assurance Programs to Support Nutritional Measurements, Anal. Bioanal. Chem., 405, 4437-4441.
- Bedner, M., Lippa, K.A., Tai, S., (2013) An Assessment of 25-Hydroxyvitamin D Measurements in Comparability Studies Conducted by the Vitamin D Metabolites Quality Assurance Program, Clin. Chim. Acta, 426, 6-11.
- Phinney, K.W., Bedner, M., Tai, S.S.-C. , Vamathevan, V.V., Sander, L.C., Sharpless, K.E., Wise, S.A., Yen , J.H., Schleicher, R.L., Chaudhary-Webb, M., Pfeiffer, C.M., Betz, J.M., Coates, P.M., Picciano, M.F., (2012) Development and Certification of a Standard Reference Material for Vitamin D Metabolites in Human Serum, Anal. Chem., 84 (2), 956–962.
- Bedner, M., Phinney, K.W., (2012) Development and Comparison of Three Liquid Chromatography-Atmospheric Pressure Chemical Ionization/Mass Spectrometry Methods for Determining Vitamin D Metabolites in Human Serum, J. Chromatogr. A, 1240, 132-139.
- Tai , S.S.-C., Bedner, M., Phinney, K.W., (2010) Development of a Candidate Reference Measurement Procedure for the Determination of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in Human Serum Using Isotope-Dilution Liquid Chromatography/Tandem Mass Spectrometry, Anal. Chem., 82, 1942-1948.
- Phinney, K.W., (2008) Development of a Standard Reference Material for Vitamin D in Serum, Am. J. Clin. Nutr., 88(2), 511S-512S, Suppl. S.

