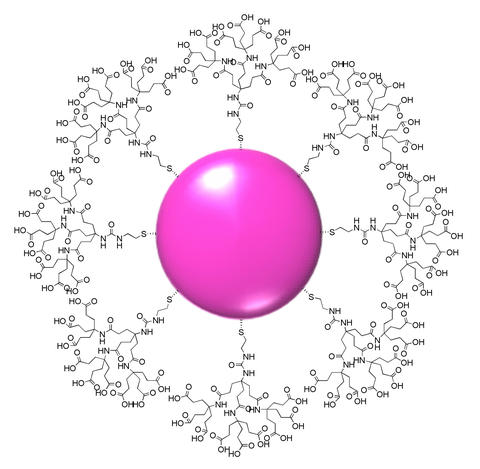
Sketch of a typical gold nanoparticle encased in dendrons. A single sulfur atom at the 'root' of each multiply branched dendron anchors it to the gold nanoparticle at the center. NIST and NCI/NCL researchers are studying the tiny constructs as a test bed and basic vehicle for many possible biomedical applications.
Gold nanoparticles are becoming the ... well ... gold standard for medical-use nanoparticles. A new paper by researchers from the National Institute of Standards and Technology (NIST) and the National Cancer Institute's Nanotechnology Characterization Laboratory (NCL) proposes not only a sort of gold nanoparticle "testbed" to explore how the tiny particles behave in biological systems, but also a paradigm for how to characterize nanoparticle formulations to determine just what you're working with.
Prospective uses of gold nanoparticles, says NIST chemist Vince Hackley, include high-precision drug-delivery systems and diagnostic image enhancers. Gold is nontoxic and can be fashioned into particles in a range of sizes and shapes. By itself, gold doesn't do much biologically, but it can be "functionalized" by attaching, for instance, protein-based drugs along with targeting molecules that cluster preferentially around cancer cells. The nanoparticles are generally coated as well, to prevent them from clumping together and to avoid rapid clearance by the body's immune system.
NCL's Anil Patri notes that the coating composition, density and stability have a profound impact on the nanomaterial safety, biocompatibility (how well the nanoparticles distribute in the body), and efficacy of the delivery system. "Understanding these parameters through thorough characterization would enable the research community to design and develop better nanomaterials," he says.
To facilitate such studies, the NIST/NCL team set out to create a nanoparticle testbed—a uniform, controllable core-shell nanoparticle that could be made-to-order with precise shape and size, and to which could be attached nearly any potentially useful functionality. Researchers then could study how controlled variations fared in a biological system.
Their trial system is based on regularly shaped branching molecules called dendrons, a term derived from the Greek word for "tree." Dendron chemistry is fairly new, dating from the 1980s. They're excellent for this use, says NIST researcher Tae Joon Cho, because the individual dendrons are always the same size, unlike polymers, and can readily be modified to carry "payload" molecules. At the same time, the tip of the structure—the "tree's" trunk—is designed to bond easily to the surface of a gold nanoparticle.
The team made an exhaustive set of measurements so they could thoroughly describe their custom-made dendron-coated nanoparticles. "There aren't a lot of protocols around for characterizing these materials—their physical and chemical properties, stability, et cetera," Hackley says, "so, one of the things that came out of the project is a basic series of measurement protocols that we can apply to any kind of gold-based nanoparticle."
Any single measurement technique, he says, is probably inadequate to describe a batch of nanoparticles, because it likely will be insensitive to some size ranges or confused by other factors—particularly if the particles are in a biological fluid.
The new NIST/NCL paper provides the beginnings of a catalog of analysis techniques for getting a detailed lowdown on nanoparticles. These techniques include nuclear magnetic resonance spectroscopy, matrix-assisted laser desorption/ionization mass spectrometry, dynamic light scattering, ultra-violet/visible spectroscopy and X-ray photoelectron spectroscopy. The dendron-coated nanoparticles also were tested for stability under "biologically relevant" conditions of temperature, acidity and some recognized forms of chemical attack that would take place in the bloodstream. In vitro biological tests are pending.
The work was funded in part by the National Cancer Institute, National Institutes of Health.

