Remember that pair of gold electroplated earrings you bought years ago at the mall? (Oh yes, you do.) Key to crafting their allure was the ability to place an ever-so-thin layer of valuable metal atop a less costly base material. This same strategy will be central to building the "engines" of future hydrogen-powered cars, and scientists at the National Institute of Standards and Technology (NIST) have developed a way to do it more effectively with metals rarer than gold.
The new method could yield better and more economical options for creating hydrogen fuel.
Fuel cells work by splitting off hydrogen from a larger source molecule—preferably by passing an electric current through water, a process called electrolysis. As you might recall from high school chemistry, splitting molecules is easier if you've got the right catalyst. For electrolysis in fuel cells, platinum often fills the bill.
Immerse two platinum electrodes—or conductors—in water, add electricity, and you've got pure hydrogen gas at one electrode surface and oxygen at the other. Reverse the process by feeding hydrogen and oxygen to the respective electrodes and the result is a fuel cell that produces electricity, leaving only pure water as a by-product.
Another metal right near platinum in the periodic table, iridium, is even more adept at producing oxygen, but it is extremely rare. Unfortunately, using either one of these precious metals can break the bank. Even in the very small amounts needed, the high cost of the catalyst and scaling of the associated fabrication processes currently limit the practicality of a hydrogen economy.
Obviously, simple and inexpensive methods for making platinum and iridium coatings as thin as possible while maintaining—or better yet, enhancing—their performance would be welcome, as would alternative materials for use as catalysts.
That's where NIST's efforts come in. In 2012, team members found a way to deposit one-atom-thick layers of platinum onto a gold base. The findings were encouraging, and the group subsequently extended the method to deposit the metal catalyst on less expensive substrates, such as nickel.
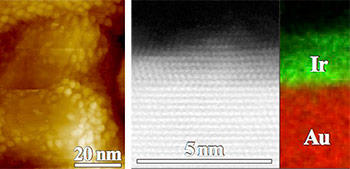
The team then set their sights on iridium, a more efficient, but even rarer catalyst.
"We wanted to see whether we could extend the rapid, one-step 'wet' atomic-layer-deposition method to other metals and substrates," says NIST materials scientist Tom Moffat. "So, we've been exploring different catalyst-substrate combinations to see if we can optimize bimetallic interactions to further improve performance, and thereby, broaden the range of options for designers of fuel cells and related devices."
The effort paid off, as the team now has shown their technique can rapidly electroplate ultrathin layers of several metals in the periodic table—known as the platinum group—onto gold and more affordable base metals, such as nickel.
For example, even a minimal amount of iridium, the most attractive candidate in the group, proved effective in producing hydrogen fuel and the oxygen needed to burn it with the ultrathin films matching or exceeding the best reported activity metrics for bulk iridium electrodes.
S.H. Ahn, H. Tan, M. Haensch, Y. Liu, L.A. Bendersky and T.P. Moffat. Self-terminated electrodeposition of iridium electrocatalysts. Energy and Environmental Science, DOI: 10.1039/C5EE02541A, Oct. 30, 2015

