Summary
This project provides measurement solutions that probe the surface and internal structure of polymer membranes used in water purification, and correlate that structure to the transport of water and other species through the membrane. Our methods are focused on elucidating the role of roughness, surface charge, surface chemistry, crosslink density and monomer chemistry on the interfacial and internal dynamics of the membrane. With this knowledge, industry will better understand the performance of these membrane materials as well as identify essential features to enable next-generation, energy-efficient, high-flux membranes.
Description
Impact
Access to affordable, clean water is vital to the nation's economic growth and security. Polymer-based membrane separation technologies based on reverse osmosis, forward osmosis and nanofiltration will play an increasingly critical role in the production of clean, safe water. In order to remain competitive, these membrane technologies must demonstrate improvements in water flux and reductions in energy consumption.
However, the membrane community lacks a "quantitative understanding of the connections between the molecular structure of the membrane and water permeability."1 Such an understanding will guide innovative designs of next-generation water purification membranes with improved performance and reduced energy demands.
Approach
Our team is developing measurement methods to correlate membrane structure and dynamics to performance (flux and selectivity) and improve reproducibility and comparability of measurements on these materials. We have developed an approach for synthesizing model crosslinked polyamide membranes based on molecular layer-by-layer (mLbL) deposition, which enables precise control over network structure, surface chemistry, residual charge, roughness and film thickness as well as placement of specific functional groups at defined locations within the network.
Armed with these model membranes as a reference, we apply our expertise in advanced surface analytical tools such as X-ray photoelectron spectroscopy, near-edge X-ray adsorption spectroscopy fine structure and atomic force microscopy to assess the impact of surface structure on permeability and transport of water and solutes across the water-membrane interface. We also employ scattering techniques such as X-ray/neutron reflectivity and high-flux backscattering to measure the molecular topology and chain motions of the membrane network, which will be correlated to the diffusion and transport of water and solutes through the membrane.
1Society News, MRS Bulletin 37, 163, (2012).
Major Accomplishments
Molecular Layer-by-Layer Deposition of Model Polyamide Active Layers
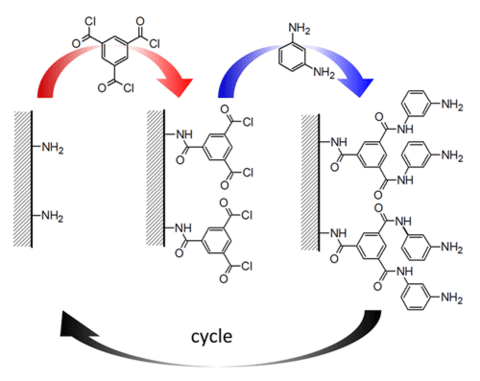
To form the polyamide active layer used in reverse osmosis membranes, interfacial polymerization of trimesoyl chloride (TMC) and m-phenylene diamine (MPD) occurs at an organic-water interface. Although effective, the rapid polymerization rate and unstable reaction conditions produce films with rough surface structures and chemical heterogeneity. Therefore, quantification of membrane structure-property relationships is difficult, since changes in synthesis conditions alter multiple properties, and film roughness prevents depth profiling analysis techniques.
To address this measurement need, we developed an approach based on a molecular layer-by-layer (mLbL) assembly process, alternating single reactions of TMC and MPD, for producing model polyamide active layers (Figure 1).
This process affords exquisite control of membrane topology, monomer placement, surface chemistry and film thickness. Furthermore, the resulting chemistry at the mLbL surface provides a facile route for subsequent chemical modification of the membrane surface, which allows for systematic studies of the effect of surface chemistry and morphology on permeability and transport of molecules across the membrane interface. We control film thickness directly by the number of sequential reaction steps performed. The resulting films exhibit more than an order of magnitude reduction in surface roughness as compared to conventional interfacial polymerization, thus enabling advanced measurements of molecular topology and transport (Figure 2).
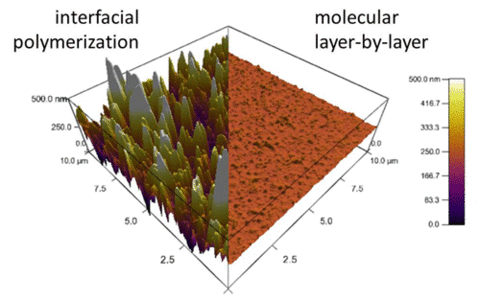 |
Figure 2. Atomic force microscopy images comparing mLbL film to its interfacially polymerized counterpart. |
Mechanical Properties of Commercial Polyamide Active Layers
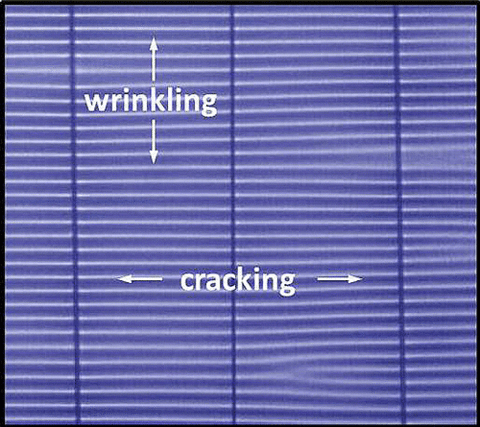
During use, the polyamide active layer can undergo chemical degradation due to operating at high pressures and temperatures, as well as the introduction of chlorine to prevent membrane fouling. This chemical degradation can lead to changes in the mechanical properties of the membrane active layer that eventually lead to membrane failure. There is, however, a dearth of information concerning the full mechanical spectrum of these types of thin layers due to the challenge of measuring materials at the nanometer scale.
In response to this need, we established and validated a measurement method based on wrinkling-cracking phenomena that allows unambiguous measurements of three fundamental mechanical properties in nanoscale thin film geometries, including elastic modulus, strength and fracture strain. In this approach, we transfer the active layer from commercial membranes onto a soft, elastomeric substrate and apply an increasing uniaxial tensile strain (ε). At relatively low strains, a critical point is reached where a periodic wrinkling pattern appears parallel to the applied strain with a well-defined wavelength (λ). As described previously and extensively investigated, this wavelength can be related to the elastic modulus or stiffness of the film. At higher strains, the film begins to crack orthogonal to the strain direction, showing multiple cracks with roughly equal spacing (d) that decreases with increasing strain. By monitoring the average crack spacing as a function of strain, we can deduce both the fracture strength and onset fracture strain of the film. The ability to measure these three material properties provides key figures of merit for assessing the mechanical characteristics of thin films and membranes (Figure 3).