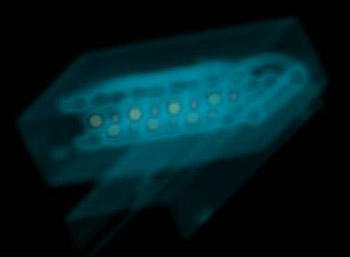
X-ray image of a length standard for medical CT. The standard is made of six rows of polytetrafluoroethylene (PTFE) balls held in place with plastic spacers. (Center of image, assembly is approx. 96 mm long.) Using a CT image, it is possible to determine the spacing of the centroids of these balls to within approximately 0.1 mm.
The idea of probing the body's interior with radiation stretches back to experiments with X rays in the 1800s, but more than a century later, images taken with radiological scans still are not considered reliable enough to, for example, serve as the sole indicator of the efficacy of a cancer treatment. Lisa Karam, a biochemist at the National Institute of Standards and Technology (NIST) and a few dozen of her colleagues across North America have set out to change that.
The group of radiology specialists from a number of institutions has recently published a pair of papers that Karam describes as part of a major effort to turn medical imaging—CT scans, PET scans, MRIs, X rays and the like—into a quantitative research tool, one that produces reliable numbers. Karam says her hope is that the group's efforts will enable scientists to determine whether a new drug or treatment method is working within weeks rather than months or years, thereby cutting down on the time it takes to get an effective new therapy approved for patients.
"Let's say doctors are studying an experimental drug that might destroy lung tumors," says Karam, "CT scans might show that patients' lung tumors shrink after a few weeks on the drug, but regulators would not accept this as evidence that the drug works because legitimate concerns exist about other variables that might be responsible for the apparent change in the image. What we want to do is get control over enough of those variables so that these concerns will fall away."
The many coauthors of the two papers are members of a subgroup of the Radiological Society of North America called the Quantitative Imaging Biomarker Alliance (QIBA). NIST's primary role, Karam says, is in helping to get control of anything involved in the imaging process that has to do with measurable physical values, from the radiation beams to the properties of the tissues being imaged.
In the example of lung tumors, Karam says, one problem is that lung tissue has a spongy quality and moves during the scan as a patient breathes. "If a patient moves a couple of millimeters during a scan, it can affect your measurement of a 10-mm tumor," she says. "So we are creating a plastic 'benchmark' that can be used to quantify this movement and account for it in the image analysis. We also are creating objects of defined density to calibrate scanners, so you can be sure of what you are measuring even if the surrounding tissue's density varies."
The first of the two papers discusses how the radiology community can best come together to make quantitative imaging the norm in medicine; the second, how to overcome longstanding issues that have discouraged the health care community from supporting the idea. Karam says more papers from QIBA are in the works: a near-term goal is to offer a method of handling data so that it can be useful in evaluating treatment methods.
"We're trying to develop a milieu for medical imaging," Karam says. "We want to show the world that a medical image can be a useful tool for medical decision making. It can give you hard numbers you can take to your insurance company and use as justification to get treatment."

