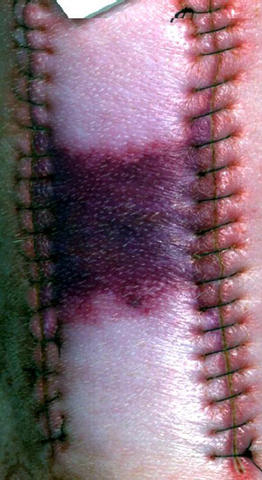
David Allen takes readings using a NIST standard reflectance diffuser prior to scanning a wound area on an anesthetized pig.
Clinicians who treat severe wounds may soon have powerful new diagnostic tools in the form of hyperspectral imaging (HSI) devices, calibrated to new NIST standard reference spectra, which will provide unprecedented perspective on the physiology of tissue injury and healing.
For example, a key factor in wound healing is the amount of oxygen in the tissue – a function of the gradual re-establishment of small blood vessels severed in the injury. At present, that process can't be assessed without biopsies or transcutaneous techniques limited to a single point.
Many physicians would prefer to use completely non-invasive HSI methods that have become available in the past decade to measure perfusion and other variables that determine outcomes over large areas.
"The potential of HSI is there, its utility has been demonstrated, and people are aware of it," says David Allen of PML's Sensor Science Division. "But HSI isn't being used routinely in the clinic yet. Why? One big reason is that standards aren't in place. Individual researchers doing their own experiments show positive results. But when you start comparing an instrument in one lab to an instrument in another, the data are typically inconsistent." Factors limiting repeatable results include biological variability (variations in skin pigmentation, tissue density, lipid content, and blood volume changes), and sensor variability related to calibration and best practices in the measurement protocol. While the biological variability is beyond researchers' control, the sensor variability can be minimized.
In pursuit of that goal, Allen and colleagues have now produced the first prototype "digital tissue phantoms" derived from bench-top simulations and in-vivo wound imaging. Phantoms are objects that are deemed reasonably equivalent proxies for the body or its components. The new PML digital tissue phantoms (DTPs) are a set of specific spectral signatures and images that correspond to different states of hemoglobin oxygenation due to ischemia (inadequate blood flow) which ultimately result in cell death due to oxygen deprivation.
Ultimately, more extensive and clinically validated versions of the phantoms can be used to calibrate spectral imaging devices of various kinds. Those devices will be able to detect the telltale spectral evidence of ischemia, revascularization, assorted pathologies, and other conditions suggestive of tissue viability at a wound site, on a microvascular scale – even during surgery.
But in order for that to happen, there must be a well-characterized and clinically validated correspondence between particular spectral signature and particular tissue conditions. Allen and scientists from Ron Xu's group at the Ohio State University (OSU) Biomedical Engineering Dept, recently took a major step in that direction by imaging carefully manipulated ischemic wounds in an anesthetized pig. "This is the first time anybody has looked at a porcine skin flap animal model hyperspectrally," Allen says. "The collection of the hyperspectral data for use as a reference is a small but very significant milestone."
On two areas of the animal's back, flaps of skin were raised, silicone-plastic sheets were placed beneath the skin to inhibit reperfusion, and the incisions were closed. On adjacent areas were a skin flap without plastic sheets and an untreated control area. Allen scanned all the areas with a highly sensitive hyperspectral imager, using illumination from a standard broadband light source. In this work, a typical scan encompassed 240 different wavelengths, spaced about 2 nm apart, ranging from 400 nm to 880 nm. As a reflectance reference, each image also included light from a NIST-traceable standard diffuser. Readings at each wavelength were then stacked into "data cubes" for each scanned position.

"We really nailed it," Allen says. "We found that we can reproduce the tissue spectrum – including the oxygenation level of hemoglobin – to within one standard deviation, well within the natural variability of the tissue."
The work is the latest development in an initiative that began about five years ago at the behest of former NIST Director William Jeffrey. "He met some people in the field, biomedical engineers and researchers who had begun doing this kind of work, and asked how NIST could help," Allen says. "This community was excited to partner with NIST in working towards advancing this technology."
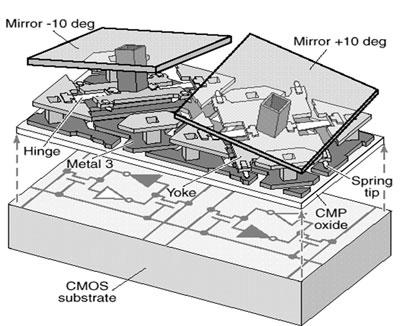
Soon thereafter, Allen's group applied for, and received, competitive NIST funding to develop standards for the nascent field. The principle investigators included are Toni Litorja, Jeeseong Hwang, Antonio Possolo, Eric Shirley and David Allen. One part of that award supported use of a PML device called the hyperspectral image projector (HIP), developed by Joe Rice and others at NIST, which reproduces complex spectral-spatial images very accurately by precision control of digital micromirror devices. (See Figure 3.)
When medically significant hyperspectral scenes are projected, they are referred to as digital tissue phantoms. They allow realistic medical scenes to be produced repeatedly without the variability and expense that would occur if the medical procedure was repeated every time that a hyperspectral image was evaluated. Already Allen's group has been able to generate HIP-projectable spectral signatures that correspond to different levels of oxygenation in tissue.
Currently, accumulating the data for DTPs is a time-consuming process: Each scan takes from tens of seconds to a minute or more. "That's the state of the technology right now," Allen says. "But in the future, we'll have 'snapshot' hyperspectral scans for real-time imaging, including video. Eventually, we want to get to the point at which you can see the blood perfusing through the tissue."
As more measurements accumulate, Allen says "we'll be able to collect a standard set of these data cubes that are well known and well characterized, and make them available as a kind of library with an indefinite shelf life. Users could then come here with their instruments, view our projections, and see if they get the same results. We have done the same sort of thing in the past, providing 'ground truth' data for satellite sensors that measure ocean color." Because the spectra are in digital form, they can be reproduced indefinitely and identically.
So far, Allen's group has had productive collaborations with OSU and at the University of Texas (UT) at Southwestern Medical Center, where scientists continue to make HSI measurements of various surgical procedures. More will join the effort. "We need to repeat the procedure in different labs using different approaches," Allen says, using both in-vivo sources and bench-top apparatus that can be tuned to simulate the reflectance signature of different organs at different oxygenation levels. In the long run, these studies will make it possible for HSI to be used as a non-invasive diagnostic tool that will provide rapid results with a much greater ability to discriminate between healthy and diseased tissue. Some examples include burns, chronic wounds, and tissue margins for surgical removal of tumors. Establishing the measurement uncertainties will help guide researchers in determining the relationship between the optical measurements and what is clinically significant.
And there are other, quite different, potential uses as well. In addition to optical medical imaging, Allen is also investigating HSI's potential in areas including environmental and defense applications such as diseases of coral reefs and the detection of hidden explosive devices. Results to date are highly promising.