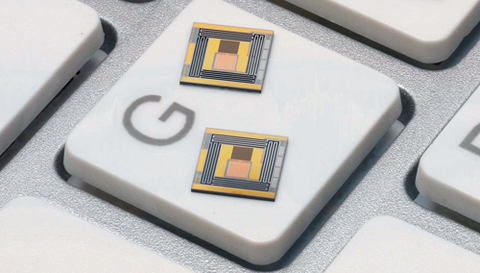
Two of the TES-based devices sitting on one key of a computer keyboard. The brown rectangle at the top is the mount upon which the gold-and-plutonium sample is placed. It is connected to the TES (pink lower rectangle).
A collaboration between NIST scientists and colleagues at Los Alamos National Laboratory (LANL) has resulted in a new kind of sensor that can be used to investigate the telltale isotopic composition of plutonium samples – a critical measurement for nuclear non-proliferation efforts and related forensics, as well as environmental monitoring, medical assays, and industrial safety.
The novel device, based on "transition edge" sensor technology developed at NIST,* is capable of 10 times better resolution than all but the most expensive and time-consuming of current methods, and reduces the time needed for sample analysis from several days to one day. Researchers from NIST and LANL describe the new design and its results in the journal Analytical Chemistry.
Plutonium (Pu), highly radioactive and extensively employed in nuclear weapons and reactors, has many isotopes, and any trace sample contains slightly different proportions. Pu-239 is the main constituent of both weapons-grade and power reactor-grade plutonium; Pu-240 is the principal minority isotope.**
Analyzing the exact mass ratio of the two in a sample reveals important information about the material's origin, processing history, safety, and possible intended use. A key potential application in nuclear forensics is the attribution of a radiological event.
Until now, high-resolution mass-ratio results have only been possible by using mass spectrometry, a complicated procedure in which atoms of different masses travel different trajectories through a magnetic field. The more common, but less precise, method is to use solid-state detectors which absorb the alpha particles *** emitted by the radioactive sample and record their energy. Each isotope emits alphas with slightly different specific energies. So measuring the number of alpha particles at each energy level reveals the content of the sample.
"That sounds simple, but the issue is complicated by the fact that each isotope can have multiple decay modes that can lead to two, three, even four or more alpha particle energies per isotope," says Joel Ullom, leader of NIST's Quantum Sensors Project. "Even the best solid-state detectors cannot fully resolve all of the alpha particle energies, so the analysis of data from solid-state detectors relies on extensive knowledge of all of the overlapping alpha particle energy lines."
The TES-based design has better intrinsic energy resolving power than solid-state sensors. But that is not the only advantage. "Since the TES is a thermal sensor, we can incorporate the radiological material directly into the sensor," says NIST project scientist Dan Schmidt. "The TES sensor can measure the total energy produced by each decay. This eliminates all of multiple alpha particle energies per isotope seen by conventional solid-state detectors, potentially greatly simplifying the analysis."
Transition-edge sensors (TES) are very small superconducting devices through which a trickle of current runs without resistance as long as the device stays below a critical temperature. Above that temperature, the sensor loses its superconductivity and reverts to normal electrical resistance. The device is cooled to the point at which it is on the edge of the transition between those two conditions.
When a photon or particle deposits energy into the TES (or a surface attached to it), it raises the temperature of the device, superconductivity ceases, and the sudden onset of electrical resistance registers as an electrical signal.
To use the new sensor design, the LANL researchers dissolved plutonium samples in solvent and placed a few drops on a piece of gold foil about one-sixth the thickness of a human hair. The solvent evaporates, and the foil is folded up so that all the Pu is enclosed. The folded foil is then squeezed about 100 times until the particle size of the solvent residue is minimized and the Pu and gold are thoroughly mixed. As a result, the gold will absorb nearly 100% of all the energy of radiation and convert it to heat.
The gold-Pu mix is then pressed onto a mount pad attached to a TES sensor. The NIST team had to design a new kind of TES that was mechanically robust enough to withstand the bonding force. Previous TES sensors used delicate membranes; the new sensors employ silicon mechanical beams for structural support.
Alpha particles emitted by Pu-239 raise the gold mix to one temperature; those from Pu-240 raise it to a different temperature. The highly sensitive TES detects the difference and the associated electronics records the number of each type. After 21.4 hours of data at approximately one emission detection per second, plots of the data points showed two distinct energy peaks, one for each isotope, with almost no overlap.
The scientists tested their TES results against mass spectrometer analysis of the same sample material and found the results to be in good agreement.
"Obtaining high quality results on much faster time scales than mass spectrometry is extremely important," says NIST project scientist Dan Swetz. "If a radiological event occurs, the forensics will need to be completed as quickly as possible."
The new sensor design is only the latest in a long series of TES accomplishments at NIST's Physical Measurement Laboratory. "We have spent the last two decades developing transition edge sensors and their superconducting electronics," says Dave Rudman, leader of the Quantum Devices Group. "The broad uses of these sensors include telescopes studying the remnants of the Big Bang, optical photon detectors for quantum communication, x-ray spectrometers conducting applied and basic materials research, and gamma-ray spectrometers for analyzing spent nuclear fuel. While these and the new plutonium device span many orders of magnitude in energy, the underlying fundamental measurement principle is the same."
* TESs are used in a variety of applications. See, for example, New High-Resolution X-ray Spectrometer for Beam Lines, and Adding Up Photons with a TES.
** Pu-240 is present in low concentrations in nuclear weapons owing to its tendency to spontaneously fission. Concentrations are higher in reactor fuel and other uses.
*** When plutonium breaks down by fission into lighter elements, it emits alpha particles (two neutrons and two protons bound together, equivalent to a helium nucleus) as well as electrons and radiation.

