Taking Measure
Just a Standard Blog
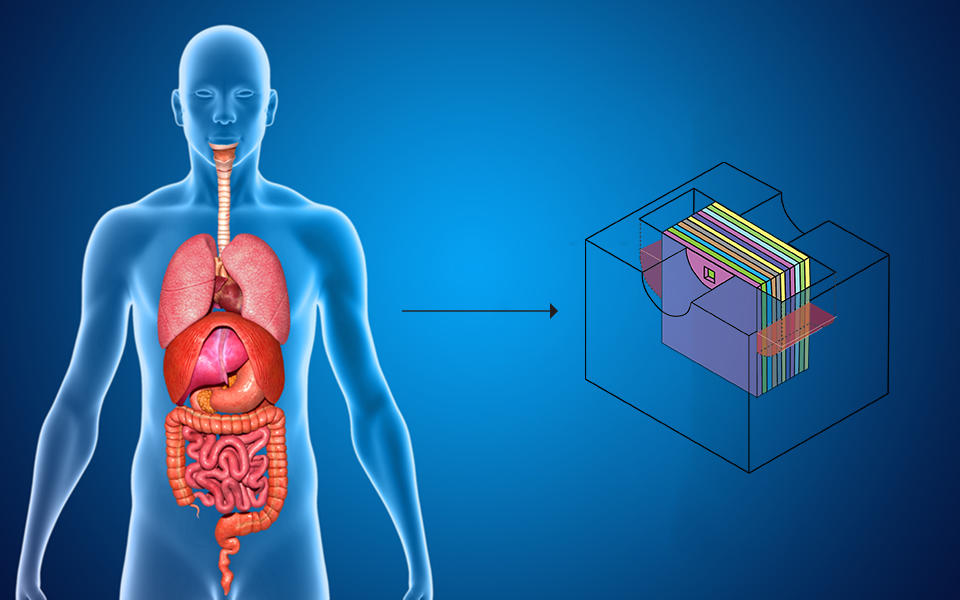
The organs of the human body can be simulated using the body cube, a schematic of which appears on the right. The body cube is a human body mimic that lets us test how drugs and other substances interact with different cells in the body, which helps researchers study their toxicity in the body in a safe way.
Growing up, I always had a love for living things, so I went off to college and got an undergraduate degree in biology. After that, I realized I wanted to specialize in an area that could help people live better lives and conquer diseases, so I decided to get a Ph.D. in biotechnology.
During my Ph.D. thesis, I learned to make tiny channels millionths or billionths of a meter in size where we can culture living cells. Cells organize themselves at that length-scale inside our bodies, so using technology that can guide cell organization at that scale in a microfluidic cell culture chamber helps make any engineered tissue behave more naturally.
While working as a life sciences liaison at the nanofabrication facility at Cornell University, I also learned about Michael L. Shuler’s work on devices that can mimic the human body. Studying biology, I was always aware that animal experiments are involved in good research, but I personally wasn’t very comfortable killing mice and treating rabbits with drugs. I actually avoided participating in such projects as much as I could. Building a human body mimic that could be used in place of animal experiments sounded like a great idea, so I decided to become a postdoc with his group.
He introduced me to the concept of devices that can culture cells from different organs of the body at the same time. They can be used to test for the efficacy and toxicity of newly developed drugs. Some drugs may not be toxic at first but can get converted to toxic molecules by the body during metabolism. Sometimes, experiments with animals can reveal such secondary toxicities, but only if the drug undergoes the same processes as it would in the human body. Often, however, the conversion of drugs follows a slightly different path in an animal, making it difficult to predict exactly what will happen in the human body. To get more information, it’s good to test the drug using something that acts like the human body, such as these multiorgan microphysiological devices.
Once I completed my training in Professor Shuler’s lab, I joined Syracuse University to teach nanotechnology for life science applications. In 2015, I was asked to come to the National Institute of Standards and Technology (NIST) to give a presentation about my work and discovered that researchers at NIST were also interested in the subject. Since NIST has a nanofabrication facility that lends itself well to developing new microfluidic devices, I decided to come to NIST and build a laboratory for the development of microphysiological systems.
While engaged in this work, I noticed that many multiorgan devices simply contain too much blood substitute to maintain realistic concentrations of the molecules that our bodies create during the processing of drugs. So, I challenged myself to develop a device that works with amounts of liquid like those found in the body. I achieved that by designing a microfluidic cell culture device where the cell culture chambers are arranged in a 3D format rather than the traditional 2D format. That allowed me to shorten the microfluidic connections between the cell culture chambers and, with that, decrease the amount of liquid needed to fill those channels. In my mind, creating more realistic blood substitute levels inside microphysiological systems is the best way to improve the reliability and certainty of toxicity measurements.
I also knew that microphysiological systems had a good chance to be of commercial use in the pharmaceutical industry. So, once I realized that we had developed a rather unique device — the body cube — I worked with the NIST Technology Partnerships Office to see if we could strike up a collaboration with a company to use it.
They told me about the FedTech Startup Studio Program, which recruits entrepreneurs and pairs them with up-and-coming research and development from federal labs, such as NIST, to launch new business ventures.
I applied for the program and was matched with a team of entrepreneurs.
It was great to explore how relevant my technology was for commercialization. It really oriented me toward real-world problems the industry has and got me looking for solutions. For example, it made me consider applications such as testing the toxicity of commercially available and industrial chemicals, or using the devices for teaching concepts about drug metabolism in the human body to students.
As the FedTech Startup Studio Program was wrapping up, the entrepreneurs I worked with formed a startup company that was among those invited to pitch its technologies to a panel of business experts. Over 200 viewers voted, and my team won the “Audience Choice” prize. It was such a great experience!
Moving forward, I’m working on generating more data for specific organ combinations that we can culture inside the body cube and integrating microfabricated sensors that allow us to make more complex measurements. Hopefully, the data can help convince both investors and customers to buy into the technology. I’m also working on a follow-up technology that will be even easier to use than the current version. My dream is to contribute to the development of microphysiological devices and help make their potential use as human body mimics a reality.






woah, cool