Taking Measure
Just a Standard Blog
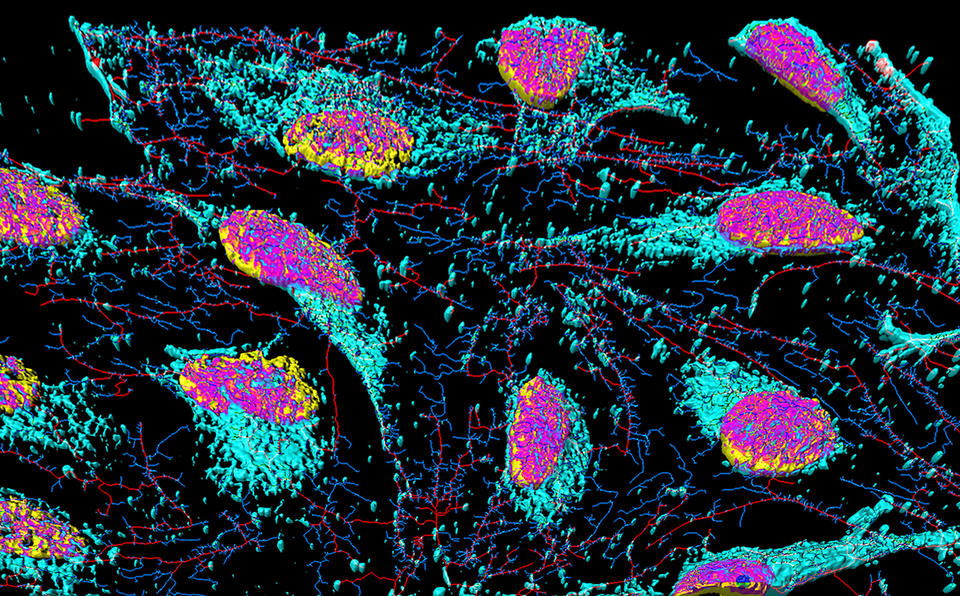
HeLa cells are stained with special dyes that highlight specific parts of each cell.
Physicists have a tendency of turning a complex problem into a simpler model that could be easily digested by mathematical equations or existing theories. Have you ever heard of “the spherical cow”? It’s a popular joke among scientists, but it captures how physicists often approach the objects they study. In short, it is easier to assume that a cow is a sphere since the calculations for a spherically shaped system are easier than those for a cow-shaped system.
Throughout my scientific career, I have dealt with idealized “spheres” like this. At the Jet Propulsion Laboratory in California, I was tasked with detecting near-Earth objects such as the Chelyabinsk meteor that exploded over the Russian city of the same name in 2013. In graduate school at Kent State in Ohio, we assumed that the rod-shaped liquid crystal molecules were cucumbers, which made it easier to visualize how they lined up next to each other.
Fast-forward to my current work at the National Institute of Standards and Technology (NIST), and now my “spheres” of interest are biological cells … of human origin.

If it wasn’t for the book entitled The Immortal Life of Henrietta Lacks by Rebecca Skloot, I would forever look at biological cells through a lens of a physicist and would see nothing more than spherical objects during data acquisition or image analysis. This influential book introduced me to Henrietta Lacks’ story.
Lacks was a Black woman born in 1920 who was diagnosed with cervical cancer when she was 30. Treatments were unsuccessful, and she died at the age of 31. Though Lacks died more than 70 years ago, her cells are still alive today. Without her awareness or consent — a serious ethical problem that protocols are in place to stop today — researchers collected cells from her cancer. They then experimented on, stored and mass-produced these “HeLa” cells, and also shared them with other scientists. They became the first immortal human cells that were ever grown in culture, meaning they continued to reproduce endlessly in a petri dish, making a tissue culture that was continuously usable and testable for medical research purposes. Her cells have contributed to major scientific advances, and we owe a great debt to her and her family.
I don’t use HeLa cells in my work, but I do use cells from humans from whom consent has been obtained. Lacks’ story forever changed my perception of my experimental samples containing living spherical objects and made me realize their connection to real people, not just where the cells come from, but also whom my work affects.
One of the cells that I utilize in my research is called Jurkat Clone E6-1, derived from the Jurkat cell line, which was established in the late 1970s from the blood of a 14-year-old male with acute T-cell leukemia. By this time, donors (or their representatives such as family members) had gained the right to specify how to use their cells. The Jurkat cells that we have obtained can only be used for laboratory research, and not in medical treatments for example. But laboratory research with these cells can lead to broad advances in science and medicine.

Using these cells, collaborators and I are aiming to come up with a better way to measure the viability of a human cell. Put simply, a viable cell is alive and able to function.
Cell viability is a critically important property for biotechnology applications, including the use of cells in medical therapies. Some medical therapies introduce live cells into the body, and in other cases, dead cells are used to evaluate therapies, so it is important to be able to detect the difference. Live and dead cell counts are used to evaluate cell growth, health and function, as well as to establish effective doses for therapeutic drugs. Biotech workers routinely evaluate cell viability to evaluate the manufacturing process and to ensure the quality and consistency of product batches for cell therapies.
Whether or not a cell is viable is determined by whether it has the following characteristics: an intact outer covering (membrane), the capacity to carry out life-sustaining chemical reactions (metabolism), and the ability to move, to reproduce or to react to stimuli.
Viability may sound like a qualitative property, one that is descriptive rather than numerical. But quantitative measurements can play a critical role in assessing this important property. The measurements we are developing can be linked, or traced back to, internationally recognized definitions of physical measurement units. In contrast, current cell viability methods lack such traceability and comparability, which become important, for example, when comparing results at different lab or manufacturing facilities or in the same facility at a later time.
We start by using one of the widely used methods for determining cell viability: pouring a blue dye called trypan blue on cells. Trypan blue penetrates and stains dead cells with ruptured membranes. Viable cells with intact cell membranes, however, are impermeable to trypan blue and remain unstained.

We use a microscope to capture images of Jurkat cells and then look at each pixel of the image to measure how much light the cell absorbs due to the dye. Since we know the absorbance properties of the dye, we can then determine the number of trypan molecules that absorb light. The number of molecules that absorb light can be expressed in moles, which is a unit of the International System of Units (SI). In this way, absorbance imaging enables traceable and comparable live and dead cell classifications. What’s more, as we found, dead cells can absorb different amounts of dye depending on the method that was used to kill them.
Together with our collaborators, we hope our methods for quantitative viability assessments in 2D and 3D cell cultures will accelerate the development of biological products for human use and advance the field of regenerative medicine, in which diseased cells, tissues or organs are repaired or replaced.
And thanks to the book about Henrietta Lacks, I have a whole different kind of appreciation for my samples. I now realize that behind each “spherical cow” that I study in the lab existed a person who was kind enough to donate a piece of themselves for the sake of scientific advances. We no longer know whose cells we are exploring as the personal identity is not disclosed, but in my mind, I respectfully thank the donor when I am handling their samples.
About the author
Related posts
Comments
Reading about Henrietta Lacks' impact on human cell research transformed my perspective as a physicist. Her legacy is truly inspiring.






Physics is very important