How Do You Measure PFAS?
![Animation shows firefighter helmet with moving visor and raindrops sliding off a raincoat, and reads: HDYMI? PFAS [FOREVER CHEMICALS]](/sites/default/files/images/2024/03/14/HDYMI_PFAS_v2_Animated_v3_web.gif)
They’re in the air, the water, the food and the soil. They’re in our carpets, cookware, cosmetics and clothes. They’ve probably made it to your blood, liver and kidneys; they’ve even been found in some of the most remote places on Earth.
They’re per- and polyfluoroalkyl substances, more commonly referred to by the acronym PFAS: a group of thousands of chemicals that contain a nearly unbreakable bond between fluorine and carbon. They’re often called “forever chemicals,” because once created, they last a very long time.
Since being invented in the 1940s, PFAS have found uses in all sorts of industries and products, from food packaging to firefighting foams to waterproof clothing to computer chip manufacturing. They’ve also saturated our environment. And they may be harming our health: Scientists have found evidence that certain PFAS can suppress the immune system and cause cancers, obesity and birth defects.
Tackling this emerging health and environmental challenge will require first overcoming a daunting measurement challenge. Forever chemicals in food, water, soil and other materials are often present in minuscule amounts. The Environmental Protection Agency, for example, has proposed limiting certain PFAS in drinking water to 4 parts per trillion — as dilute as a few drops in an Olympic-size swimming pool. The need to measure forever chemicals in such tiny concentrations has forced chemists to get creative.
Let’s say a chemist receives a sample of water or food with suspected PFAS contamination. How can they figure out what they have, and how much?
First, chemists must “train” their instruments by running them on what’s known as a standard — a commercially available concentrated solution that contains known quantities of common PFAS. The training process calibrates the equipment to ensure that measurement results for samples that contain unknown quantities will be accurate.
Chemists must also ensure they’re measuring the chemicals they intend to and not something else. For example, commercial lab equipment components such as plastic tubes are often coated with PFAS-containing materials such as PTFE — often referred to by its trade name, Teflon — to help liquids flow smoothly. That background PFAS must be accounted for or eliminated by installing PFAS-free components.
Non-PFAS chemicals can also mimic the compounds of interest and confound results. Such unwanted chemicals, known as interferents, have led to high-profile cases of mistaken identity. For example, a few years ago, the Food and Drug Administration thought it had detected PFAS in chocolate cake, triggering a spate of alarming news stories. Upon further analysis, the agency determined the cake contained no actual PFAS.
After controlling for background chemicals, scientists extract the PFAS from their sample using acid, salts, shaking and centrifuging. This concentrates the chemicals so they will show up in subsequent measurements.
Scientists then turn to a technique called chromatography, which takes advantage of the fact that lighter chemicals move faster through a column or tube than do heavier ones. Chromatography separates the various chemicals in a sample from each other.
Next, scientists feed the chemicals into a machine called a mass spectrometer. Mass spec, as it’s often called, takes advantage of the fact that electrically charged molecules move differently in a magnetic field depending on their charge and weight.
PFAS are good candidates for mass spec because they are typically electrically charged, and each has a distinct molecular weight. Mass spec results can then be matched to properties of known PFAS to pinpoint the identities — and concentrations — of the mystery compound or compounds.
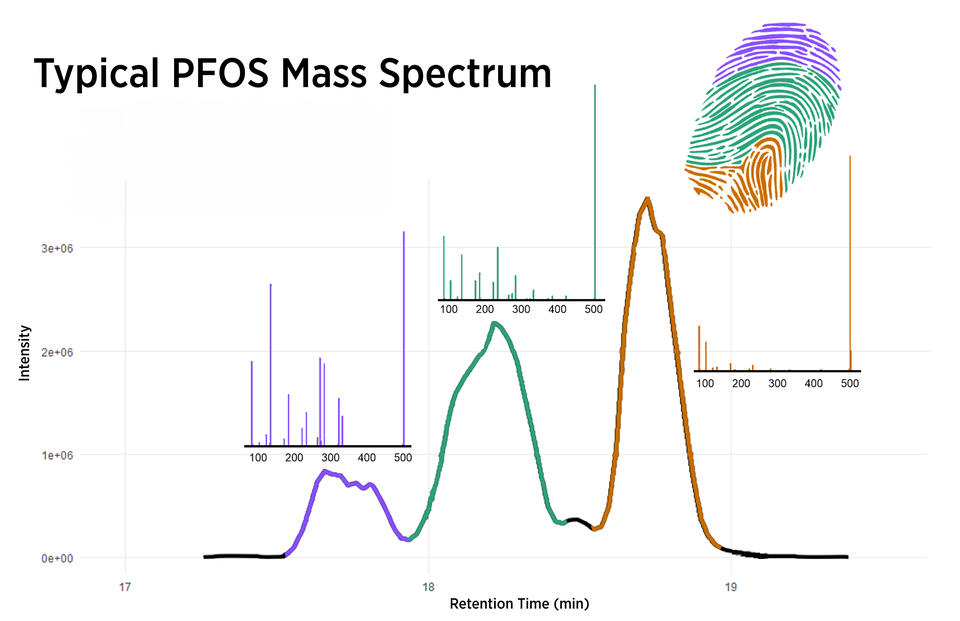
When the problem of widespread PFAS contamination emerged several decades ago, labs often produced wildly varying estimates of PFAS concentrations in samples of water and other materials. Thanks to today’s measurement techniques and standards, lab results have become much more accurate and reliable.
With new regulations for PFAS in food and water coming down the pike, far more labs will be tasked with measuring these chemicals. That makes the need for accurate and consistent measurement of forever chemicals more important than ever.
You can learn more about NIST's work measuring forever chemicals in this feature story, Finding Forever Chemicals Wherever They’re Hiding.
Learn More

