Fit-for-Purpose Viability Measurements
Cell viability assays are frequently used to characterize the health of cells in biomanufacturing and research settings. Cell viability assays can utilize different molecular, physical, and chemical markers to identify viable and non-viable cells based on user-defined criteria. Cell viability can also be evaluated on a population level, to evaluate the overall health of the culture. With a broad range of both assays and instrumentation available for evaluating cell viability, approaches are needed to design viability assays that are fit for the intended purpose. This is especially critical when viability assays are being utilized to support decision making in cell-based manufacturing and product release.
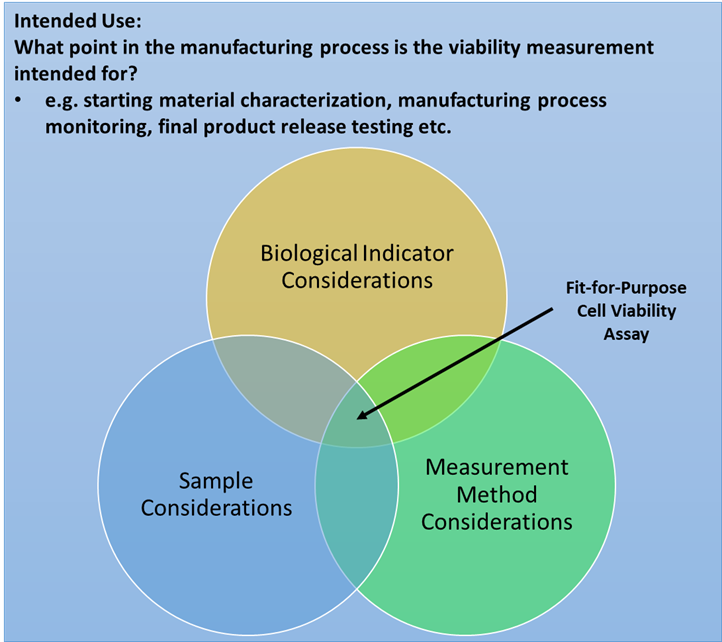
Based on a NIST-led Cell Viability Workshop, we have developed a framework for conceptualizing key considerations when designing a fit-for-purpose cell viability assay (Figure 1). This framework includes considerations for sample specific properties that may affect a cell viability assay (e.g. sample stability, heterogeneity, presence of debris), identification of appropriate biological indicators that are relevant to the intended use, and measurement method considerations including requirements for measurement performance (e.g. precision, accuracy, specificity etc.) and practical considerations (e.g. cost per assay, level of automation etc.).
Approach for Establishing Fit-For-Purpose Cell Viability Measurements that are Sensitive to Proliferative Capacity in a Model System
For cell viability results to be meaningful, they should be indicative of the utility of the cells in the next steps of a bioprocess or be relevant to the safety and effectiveness of a final product. We are currently developing approaches to identify fit-for-purpose cell viability assays which can predict changes in cell population proliferation kinetics following a stress or injury (Figure 2). We use Jurkat cells as a representative model cell line for T-cells used in cell therapy manufacturing and proliferation as a required property of the cells for the manufacturing process. Through exposing Jurkat cells to mild injury using heat (44°C), for different amounts of time, we can generate a graded proliferation response. We measure resulting viability post-injury with a selection of cell viability assays and monitor the subsequent proliferation kinetics of the population. We then draw relationships, where possible, between viability measurements taken at different timepoints post-injury and viability results using a suite of viability assays.

A critical aspect of this approach is the ability to generate systematically varied cell samples with graded functional response (Figure 3A). Different viability assays provide different levels of information about the cell population proliferation kinetics at different timepoints (Figure 3A and 3B). For example, AO/DAPI cell viability assays conducted shortly after a heat treatment do not show any trend with decreasing proliferation rates of paired cell samples (proliferation rate is characterized by the T2 inflection point derived from the Gompertz model fit of time course cell concentration data). Instead, AO/DAPI measurements taken 24 h after initial heat treatment, are predictive of changes in proliferation rate as represented by shifts in the second inflection point T2.

This analysis demonstrates an approach for designing fit-for-purpose cell viability assays that may help to indicate future potential of a cell sample. The modeled relationships between the desired proliferation outcomes, and the measured cell viabilities post injury can be further used to establish meaningful specifications for cell viability in a manufacturing process.
Contacts
-
(301) 975-8595
-
(301) 975-4370

