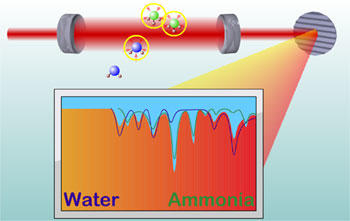
The new JILA technique uses infrared laser light in many different colors, or frequencies, to identify trace levels of different molecules at the same time. For example, water molecules blue) and ammonia molecules (green) absorb light at very specific characteristic frequencies. The pattern of frequencies absorbed forms a "signature" for identifying the molecules and their concentrations.
Boulder, Colo. -- Physicists at JILA have designed and demonstrated a highly sensitive new tool for real-time analysis of the quantity, structure, and dynamics of a variety of atoms and molecules simultaneously, even in miniscule gas samples. The technology could provide unprecedented capabilities in many settings, such as chemistry laboratories, environmental monitoring stations, security sites screening for explosives or biochemical weapons, and medical offices where patients' breath is analyzed to monitor disease.
Described in the March 17 issue of Science, the new technology is an adaptation of a conventional technique, cavity ring-down spectroscopy, for identifying chemicals based on their interactions with light. The JILA system uses an ultrafast laser-based "optical frequency comb" as both the light source and as a ruler for precisely measuring the many different colors of light after the interactions. The technology offers a novel combination of a broad range of frequencies (or bandwidth), high sensitivity, precision, and speed. A provisional patent application has been filed.
JILA is a joint institute of the National Institute of Standards and Technology (NIST), a non-regulatory agency of the U.S. Department of Commerce, and the University of Colorado at Boulder.
"What a frequency comb can do beautifully is offer a powerful combination of broad spectral range and fine resolution," says NIST Fellow Jun Ye, who led the work described in the paper. "The amount of information gathered with this approach was previously unimaginable. It's like being able to see every single tree of an entire forest. This is something that could have tremendous industrial and commercial value."
Frequency combs are an emerging technology designed and used at JILA, NIST, and other laboratories for frequency metrology and optical atomic clocks, and are being demonstrated in additional applications. NIST/JILA physicist John (Jan) Hall shared the 2005 Nobel Prize in physics in part for his contributions to the development of frequency combs. In the application described in Science, the frequency comb is used to precisely measure and identify the light absorption signatures of many different atoms and molecules.
The JILA system described in Science offers exceptional performance for all four of the primary characteristics desired in a cutting-edge spectroscopic system:
- The system currently spans 125,000 frequency components of light, or 100 nanometers (750-850 nm) in the visible and near-infrared wavelength range, enabling scientists to observe all the energy levels of a variety of different atoms and molecules simultaneously.
- High resolution or precision allows scientists to separate and identify signals that are very brief or close together, such as individual rotations out of hundreds of thousands in a water molecule. The resolution can be tweaked to reach below the limit set by the thermal motion of gaseous atoms or molecules at room temperature.
- High sensitivity—currently 1 molecule out of 100 million—enables the detection of trace amounts of chemicals or weak signals. With additional work, the JILA team foresees building a portable tool providing detection capability at the 1 part per billion level. Such a device might be used, for example, to analyze a patient's breath to monitor diseases such as renal failure and cystic fibrosis.
- A fast data-acquisition time of about 1 millisecond per 15 nm of bandwidth enables scientists to observe what happens under changing environmental conditions, and to study molecular vibrations, chemical reactions, and other dynamics.
By comparison, conventional cavity ring-down spectroscopy offers comparable sensitivity but a narrow bandwidth of about 1 nanometer. A more sensitive "optical nose" technique developed at NIST can identify one molecule among 1 trillion others, but can analyze only one frequency of light at a time. Other methods, such as Fourier transform infrared spectroscopy, provide large bandwidths and high speed but are not sensitive enough to detect trace gases.
The research at JILA is supported by the Air Force Office of Scientific Research, NIST, Office of Naval Research, National Aeronautics and Space Administration, and National Science Foundation.
As a non-regulatory agency of the Commerce Department's Technology Administration, NIST promotes U.S. innovation and industrial competitiveness by advancing measurement science, standards and technology in ways that enhance economic security and improve our quality of life.
M.J. Thorpe, K.D. Moll, R.J. Jones, B. Safdi, and J. Ye. 2006. Broadband cavity ringdown spectroscopy for sensitive and rapid molecular detection. Science. March 17.
Background: Using a Frequency Comb to Enhance Spectroscopy
Cavity ring-down spectroscopy identifies atoms or molecules by the way they absorb laser light as it is repeatedly reflected and dissipates inside a mirrored vacuum cavity.
The JILA system uses a laser that emits a broad range of colors. The laser generates about 380 million pulses per second, each lasting about 20 femtoseconds (quadrillionths of a second). The laser light is tuned to the "resonant frequency" of the cavity, such that all of the many different wavelengths of light—all "harmonics" of a single basic wave size—fit perfectly between two special mirrors. The distance between the mirrors is adjusted using tiny motors to select the resonant frequency of the cavity. The mirrors inside the laser are then rotated to match the laser frequencies to those of the cavity.
The light is repeatedly reflected inside the cavity until the laser is turned off, after which all of the energy is gradually lost in a few microseconds. If atoms or molecules are placed inside the cavity, they absorb some of the light energy at frequencies where they switch energy levels, vibrate, or rotate, and the light dissipates faster at those frequencies.
A beam of "white light" is emitted from the cavity during the dissipation process and separated into a rainbow of colors, which are detected in sets of color bands. Computer software can analyze the change in the decay time of selected channels of different frequencies simultaneously. The results are rapidly matched against a catalog of absorption signatures of known atoms and molecules.
The JILA method was demonstrated by conducting a variety of experiments with argon atoms and acetylene, water, oxygen, and ammonia molecules. The scientists demonstrated real-time, quantitative measurements of traces of gas, the frequencies and strength of signals signifying changes in energy levels, and other changes due to collisions and temperature changes inside the cavity.
For instance, the system identified a change in the acetylene signal, detected as a faster dissipation time, as the pressure of the background argon gas was increased and collisions between the gases increased. The signal resolution was sufficient to reveal spectral information that is difficult to access because it is below the physical limits set by the thermal motion of the gas molecules. In addition, analyses of water, ammonia, and oxygen demonstrated that nearly the entire 100 nm spectral range can be probed simultaneously. This combination of high resolution and broad bandwidth is unprecedented.

