Summary
The National Institute of Standards and Technology (NIST) is currently engaged in an effort to produce a Research Grade Test Material (RGTM) consisting of ribonucleic acid (RNA) encapsulated in lipid nanoparticles (LNPs). The material is intended to support the standardization of analytical techniques for characterizing LNP / messenger RNA (mRNA) drugs. Such drugs could include vaccines, such as those for COVID-19 [1,2], as well as many new drug products currently under development for applications ranging from cancer therapy to the treatment of genetic diseases. LNP/mRNA drugs are complex formulations, typically containing at least four separate lipid components, the mRNA drug substance, and buffers/excipients, which undergo assembly under carefully controlled mixing procedures. Many attributes of the resulting formulations are potentially important to drug quality and could possibly benefit from improved characterization and standardization: mRNA copy number, sequence, integrity, and encapsulation efficiency; LNP diameter, polydispersity, and morphology; transfection efficiency; lipid content/purity; and lipid/RNA chemical modifications.
Description
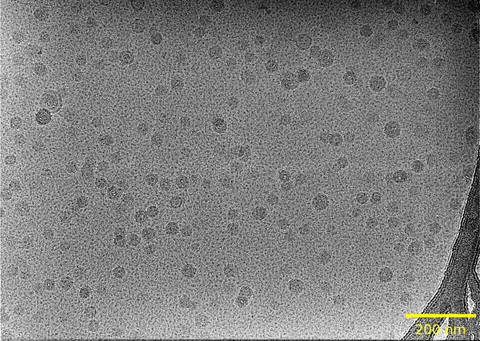
The currently planned LNP/RNA material (designated RGTM 10240) will include a fragment of the SARS-CoV-2 genome that is approximately 4 kb in length. This fragment was previously included (in unencapsulated form) as “Fragment 1” of NIST RGTM 10169. The RNA fragment is not designed for cell-based expression or in vivo activity measurements (it has no cap, modified bases, or poly-A tail), but is suitable for quantification using digital PCR, encapsulation efficiency assays, sequencing, and RNA integrity measurements.
NIST is currently recruiting participants for interlaboratory studies using the planned LNP/RNA RGTM. If your lab or institution is interested in participating in these studies, please contact us for further details. Required measurements for participants will be either LNP diameter distribution (batch DLS suggested) or RNA copy number (digital PCR suggested); participants may choose to return one or both of these measurements. In addition, we will suggest (but not require) that participants measure encapsulation efficiency. Finally, in addition to these required measurements, participants would also be welcome and encouraged to submit results from other analytical assays in which they have expertise.
References:

