Summary
Develop measurement methods that quantify the interdependent stability, transport, and redox properties of nanomaterials in biophysical multicomponent complex fluids. Sub-cellular sized particles that enter the body through a variety of pathways can elicit a toxic response. This response is strongly dependent upon the nanomaterial exposure dose and physico-chemical properties including size, aspect ratio, surface charge, and surface chemistry. In multicomponent solutions, these physico-chemical properties, solution conditions (pH and ionic strength) and other solutes (proteins and lipids) affect the stability, transport, and redox properties of nanomaterials.
Description
Engineered nanomaterials are promising for technological and medical purposes. However, molecular mechanisms of toxicology are less known. This presents a problem and barrier for future innovation and applications as new nanomaterials are developed for healthcare where particles are intentionally introduced into the bio-system. Oxidative stress represents one metric whereby nanomaterials produce oxygen-species via redox reactions. These redox processes involves transfer of electrons to/from the nanoparticle to a donor/acceptor (such as an electrode, or a even a protein). These processes are mediated by multicomponent solutions and the presence of proteins/polymers. Organic and polymer ligands bound or adsorbed to the nanomaterials may enhance or suppress the mechanisms of redox, since ligands may be insulating or conducting, or displayed at the surface with low or high grafting density, or diffuse versus compact.
Our approach focuses on unique optical and neutron scattering methods. We apply static and dynamic light scattering to measure transport properties of dilute nanomaterial solutions. Additionally, fluorescence correlation spectroscopy was developed to measure the transport properties of fluorescently-labeled nanomatials. This enables the measurement of single nanomaterials in multicomponent solutions, complementing DLS. Small-angle neutron scattering (SANS) is applied to measure the structure of nanoparticles and their complexes with adsorbed surfactants, polymers and proteins. Quartz-crystal microbalance (QCM) approaches are leveraged to characterize the adsorption of nanomaterials to taylored interfaces.
Major Accomplishments
Publications:
- RJ Murphy, D Pristinski, K Migler, JF Douglas, VM Prabhu, Dynamic light scattering investigations of nanoparticle aggregation following a light-induced pH jump, Journal of Chemical Physics, 132, 194903(2010).
- Prabhu VM, Hudson, SD, Nanoparticle Assembly: DNA provides control, Nature Materials News and Views, 8, 365-366 (2009).
Nanomaterials Environmental Health and Safety (NanoEHS) : is a priority program in MML that produces measurement approaches, standard test methods and reference materials that enable the safe and reliable use of nanoparticle materials in current and emerging technologies. The program addresses today's most important classes of nanoparticles, including carbon nanotubes, and metal and oxide particles, which are slated for use in applications ranging from biomedical technology to advanced coatings and composites.

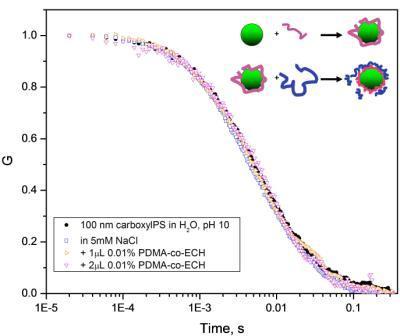
In situ nanoparticle aggregation : DLS was used to characterize the kinetics of charged carboxylate polystyrene NP aggregation in dilute solutions. The aggregation process was initiated ,in situ, using photoacid generators (PAG) in solutions. The solution pH can then be controlled by exposure to ultraviolet light without need for mixing or stirring. The kinetics of NP aggregation are sensitive to the solution pH and we find that the UV exposure dose is inversely correlated to the surface charge of the nanoparticles, effectively decreasing the electrostatic repulsion force between particles and resulting in particle aggregation. The aggregation kinetics after pH quenches for a dilute suspension of 20 nm hydrodynamic radius NP can be seen in the contour plots of the time-dependent DLS signal. The deepest quench shows diffusion-limited aggregation kinetics, while an intermediate quench shows reaction-limited aggregation kinetics (assuming validity of the Stokes-Einstein equation). These pH jump measurement can be applied to a variety of nanoparticle systems under various solution conditions.

Nanoparticle/polyelectrolyte assembly : We demonstrate that FCS measurements are sensitive to the adsorption of charged polymers (proteins, DNA are polyelectrolytes) to oppositely charged NPs. We begin with a model assembly between nanoparticle and polyelectrolyte as shown in the figure below. The fluorescently-labeled nanoparticle is measured by FCS and DLS before and after adsorption of the positively-charged polyelectrolyte which reverses the macroion complex charge. Subsequently a negatively charged polyelectrolyte is added to the solution that adsorbs and again causes surface charge inversion. This process was followed for four layers as shown by FCS. The sensitivity of FCS to the adsorption of single polymers to NP provides a high resolution measurement of the fraction of adsorbed and free solution chains. This measurement strategy will be used to study protein / NP solutions to measure the transport properties and stability of the complexes formed in situ.