Summary
New biological drugs such as peptide drugs, gene delivery cargos, and vaccines are emerging to fill pharmaceutical voids. Infrared (IR) absorption spectroscopy is a non-invasive measurement that can characterize these complex biomolecules in their original solution condition. However, conventional IR spectroscopy approaches are unsuitable for hydrated proteins due to the strong IR absorption by water. The Biomaterials group has developed a high-sensitivity IR technique based on a quantum cascade laser (QCL) and a solvent absorption compensation (SAC) method. After a series of upgrades, the system can acquire an IR spectrum covering the fingerprint range with a 1000 times higher sensitivity than conventional FT-IR spectroscopy. This breakthrough novel IR spectroscopy can become a standard spectroscopic measurement tool in biopharmaceutical and biological industries and sciences.
Description
Infrared (IR) absorption spectroscopy has been widely used as a non-invasive, label-free characterization method of chemical identification and structure for complex biomolecules. Conventional IR spectroscopy, e.g., Fourier-transform IR (FT-IR) technology, can characterize proteins and other biological molecules produced during biopharmaceutical processes. However, the interference by strong absorption by water has kept the conventional FT-IR method from characterizing low-concentration samples.
Recently, the NIST Biomaterials group developed a new optical technique called solvent absorption compensation (SAC) for quantum cascade laser (QCL)-based mid-IR absorption spectroscopy [1]. This patented method [2,3] enabled an order-of-magnitude improvement of the detection sensitivity [4] and the doubled expansion of the spectral range [5]. The project aims to make this new high-sensitivity SAC-IR technique a standard measurement tool for the non-destructive characterization of emerging biological systems, including new protein drugs and gene therapy.
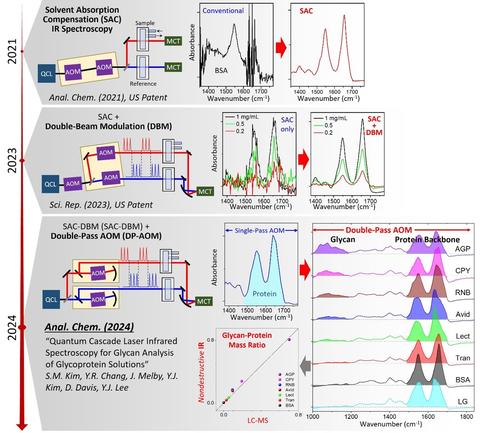
PUBLICATIONS
[1] B. Chon, S. Xu, Y. J. Lee, Compensation of Strong Water Absorption in Infrared Spectroscopy Reveals the Secondary Structure of Proteins in Dilute Solutions. Anal. Chem. 93, 2215 (2021).
[2] Y. J. Lee, Spectrum Adjuster and Producing a Pure Analyte Spectrum, US Patent Issued, 10,345,226 (2019).
[3] Y. J. Lee, Single-Detector Double-Path Intensity-Modulation Spectrometer, US Patent Published, US 2024/0167877 A1 (2024).
[4] S.-M. Kim, Y.-R. Chang, Y. J. Lee, Single-Detector Double-Beam Modulation for High-Sensitivity Infrared Spectroscopy. Sci. Rep. 13, 18231 (2023).
[5] S.-M. Kim, Y.-R. Chang, Y. J. Lee, Quantum Cascade Laser Infrared Spectroscopy for Glycan Analysis of Glycoproteins. Anal. Chem. 96, 13120 (2024).

