Summary
This project, now concluded, developed novel imaging and image analysis tools to examine individual cells and cell populations within the complex chemical environment created by neural progenitor cells. Our goals were to quantify nanoparticle incorporation by individual cells and to quantify the response of neurons exposed to sub-lethal doses of metal nanoparticles.
Description
Nanoparticle interactions with individual cells. Existing techniques to evaluate nanoparticle incorporation include ICP-AES or ICP-MS, which atomize entire samples (thousands of cells) and detect nanoparticles by quantifying the elemental composition. Bulk techniques provide general trends averaged over many cells, but cannot assess the inherent variability posed by individual cells. TEM tomography provides nanometer resolution, yet the ultimate volume analyzed is typically less than one cell. We developed correlative microscopy methods to quantify nanoparticle incorporation by individual cells.
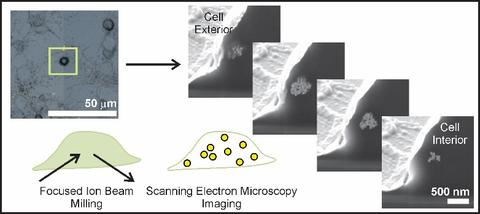
Selected publications:
Sanders, A.W., Jeerage, K.M., Schwartz, C.L., Curtin, A.E., Chiaramonti, A.N., 2015. Gold nanoparticle quantitation by whole cell tomography. ACS Nano 9, 11792-11799
https://doi.org/10.1021/acsnano.5b03815
Sanders, A.W., Jeerage, K.M., Curtin, A.E., and Chiaramonti, A.N., 2015. Localization and number of Au nanoparticles in optically indexed cells by FIB tomography. Microscopy and Microanalysis 21, 411-412
https://doi.org10.107/S1431927615002858.
Sanders, A.W., Jeerage, K.M., Schwartz, C.L., Curtin, A.E., 2014. Correlating multiscale measurements of nanoparticles in primary cells. Microscopy and Microanalysis 20, 976-977
https://doi.org/10.1017/S1431927614006606
Nanoparticles in neural progenitor cell cultures. High nanoparticle doses may drive an acute cellular response whereas lower, more realistic doses are likely to have subtle effects requiring sensitive endpoints. Monocultures that eliminate critical cell-cell interactions are another well-known limitation of in vitro toxicology studies. We developed image analysis tools to quantify the response of developing neurons within neural progenitor cell cultures to exogenous chemical or metal nanoparticle exposure.
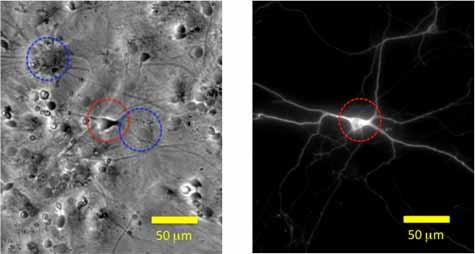
Selected publications:
Jeerage, K.M., Oreskovic, T.L., Curtin, A.E., Sanders, A.W., Schwindt, R.K., Chiaramonti, A.N., 2015. Citrate-stabilized gold nanoparticles as negative controls for measurements of neurite outgrowth. Toxicology In Vitro 29, 187-194
https://doi.org/10.1016/j.tiv.2014.10.007.
Hume, S.L., Chiaramonti, A.N., Rice, K.P., Schwindt, R.K., MacCuspie, R.I., Jeerage, K.M., 2015. Timescale of silver nanoparticle transformation in neural cell cultures impacts measured cell response. Journal of Nanoparticle Research 17, 315
https://doi.org/10.1007/s11051-015-3111-5
Jeerage, K.M., Oreskovic, T.L., Hume, S.L., 2012. Neurite outgrowth and differentiation of rat cortex progenitor cells are sensitive to lithium chloride at non-cytotoxic exposures. NeuroToxicology 33, 1170-1179
https://doi.org/ 10.1016/j.neuro.2012.06.010
Nanoparticles in tissue-mimicking hydrogels. Engineered tissue constructs provide a compliance that matches native tissue whereas polystyrene may alter cell behavior. We investigated utilization of compliant hydrogels to encapsulate nanoparticles.
Selected publications:
Mansfield, E., Oreskovic, T.L., Rentz, N.S., Jeerage, K.M, 2014. Three-dimensional hydrogel constructs for exposing cells to nanoparticles. Nanotoxicology 8 394-403
https://doi.org/10.3109/17435390.2013.790998
Hume, S.L., Jeerage, K.M., 2013. Surface chemistry and size influence the release of model therapeutic nanoparticles from poly(ethylene glycol) hydrogels. Journal of Nanoparticle Research 15, 1635

