Summary
Energy is one of the most critical problems facing the United States, with technical, economic, and public policy aspects. Our group develops state-of-the-art measurement techniques for fuel characterization, especially for the study of volatility through the measurement of distillation curves. With the resulting data, we can develop a predictive capability that is needed in all phases of design, from feedstock to engine operation. We work closely with the Thermophysical Properties of Fluids Group when making fuel property measurements and models.
Description
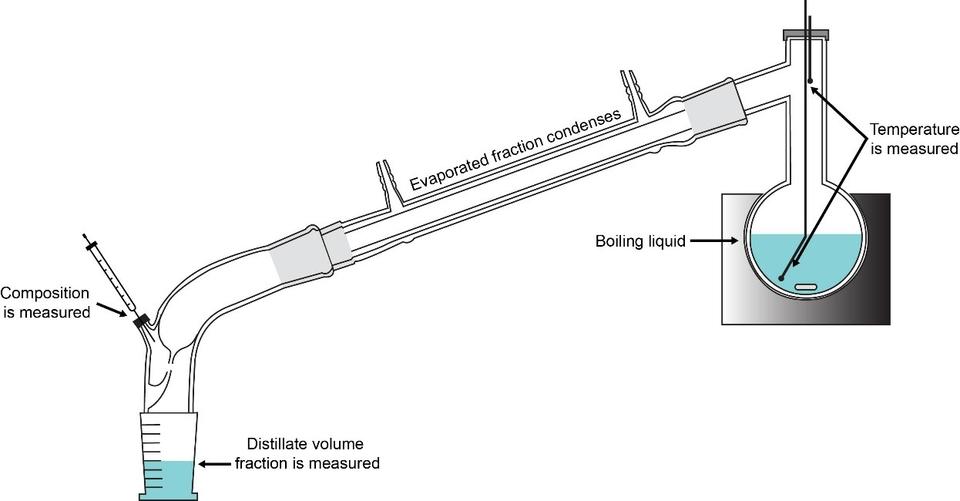
Fuel volatility and chemical characterization. We specialize in the Advanced Distillation Curve (ADC) method, developed at NIST, which is a powerful tool for the measurement of distillation curves to characterize complex fluids. We have applied this to simple hydrocarbons, gasolines, diesel fuels (including renewable fuels), aviation fuels, rocket propellants and even crude oils. The method features temperature, volume and pressure measurements of low uncertainty suitable for equation of state development, chemical analysis of each distillate fraction, and an assessment of the energy content of each distillate fraction. Our method has been adopted by other laboratories in the United States, Canada and Australia, and some of the equipment that is used in the technique is commercialized and sold by Sigma Aldrich.
Selected publications:
Harries, M., McDonald, A.G., and Bruno, T.J., 2017. Measuring the distillation curves of non-homogeneous fluids: method and case study of two pyrolysis oils. Fuel 204, 23-27, https://doi.org/10.1016/j.fuel.2017.04.066.
Harries, M., Kunwar, B., Sharma, B.K., and Bruno, T.J., 2016. Application of the advanced distillation curve method to characterize two alternative transportation fuels prepared from the pyrolysis of waste plastic, Energy & Fuels 30 (11), 9671-9678, https://doi.org/10.1021/acs.energyfuels.6b02068.
Burger, J. L., Lovestead, T. M., Bruno, T. J., 2016. Composition of the C6+ fraction of natural gas by multiple porous layer open tubular capillaries maintained at low temperatures. Energy & Fuels 30 (3), 2119-2126, https://doi.org/10.1021/acs.energyfuels.6b00043.
Burger, J.L., Harries, M., and Bruno, T.J., 2016. Characterization of four diesel fuel surrogates by the advanced distillation curve method. Energy & Fuels 30 (4), 2813-2820, https://doi.org/10.1021/acs.energyfuels.6b00107.
Bruno, T.J., Ott, L.S., Smith, B.L., Lovestead, T.M., 2010. Complex fluid analysis with the advanced distillation curve approach. Analytical Chemistry (feature article), 82: 777-783, https://doi.org/10.1021/ac902002j.
Bruno, T.J., Ott, L.S., Lovestead, T.M., Huber, M.L., 2010. The composition explicit distillation curve technique: relating chemical analysis and physical properties of complex fluids. Journal of Chromatography A, 1217: 2703-2715, https://doi.org/10.1016/j.chroma.2009.11.030.
Thermal decomposition kinetics. We have developed a batch ampoule approach to evaluate the thermal decomposition kinetics that provides (pseudo first order) rate constants, Arrhenius parameters and activation energies for simple and complex fluids. While the immediate application, in house, for these measurements is the support of our other thermophysical property measurements, the measurements are of general usefulness, and are comparable to measurements made by other approaches. We have applied this technique to finished fuels, fuel components, lubricants, and working fluids.
Selected publications:
Urness, K.N., Gough, R.V., Widegren, J.A., Bruno, T.J., 2016. Thermal decomposition kinetics of polyol ester lubricants. Energy & Fuels 30(12): 10161-10170, https://doi.org/10.1021/acs.energyfuels.6b01863.
Widegren, J.A. and Bruno, T.J., 2011. Thermal decomposition kinetics of kerosene-based rocket propellants. 3. RP-2 with varying concentrations of the stabilizing additive 1,2,3,4-tetrahydroquinoline. Energy & Fuels, 25(1): 288-292, https://doi.org/10.1021/ef101376k.
Widegren, J.A., Bruno, T.J., 2008. Thermal decomposition kinetics of the aviation fuel Jet-A. Industrial & Engineering Chemistry Research, 47: 4342-4348, https://doi.org/10.1021/ie8000666.

