Taking Measure
Just a Standard Blog
5 Concepts Can Help You Understand Quantum Mechanics and Technology — Without Math!
If you’ve heard or read about quantum mechanics, you may have seen it described as “weird.” Even the great Albert Einstein — one of the founders of quantum mechanics — called certain aspects of the theory “spooky.”
With its wave-like particles and particle-like waves, quantum mechanics certainly challenges our intuitions of how the world works. Accepting what is counterintuitive to us — while striving to learn more — is a very important part of science!
Quantum can seem intimidating because it deals with the granular and fuzzy nature of the universe and the physical behavior of its tiniest particles that we cannot see with our eyes. Just because we haven’t experienced the world of quantum the way we can see the effects of gravity doesn’t mean quantum has to be “weird” or “spooky.”
The founders of quantum mechanics may have thought it was “weird” because it was different from the physics they were used to. But that was more than 100 years ago. Quantum just is the way it is!
I’m passionate about flipping the script on quantum and making it accessible to all.
In this blog post, I will attempt to normalize quantum mechanics by drawing analogies to concepts you may already know and understand.
I will also try to explain the five things that I have noticed confuse people about quantum mechanics. (Don’t worry; no math will be required!) You probably don’t need to understand quantum mechanics in depth, but I hope this will help you think about it and how it applies to your life.
Quantum in action
Before the early 2000s, computers did not exhibit quantum behavior. But as technology advanced and transistors in computers got smaller (now as small as 5 nanometers, which is 5 billionths of a meter!), they started to show quantum behavior. Quantum behavior limits how small transistors can be and how fast computers can compute because it makes transistors “pesky” in that they don’t exhibit the predictable behavior that engineers want. For this reason, computers now operate on multiple “cores” to help increase computing speed and power.
The Wonderful World of Quantum
When you zoom in on matter at the quantum scale, nature gets granular. At this scale, we find tiny particles such as:
- Photons: particles of light that have no mass or charge.
- Electrons: subatomic particles that make up the atom, carry electricity and have charge and mass.
- Quarks: the building blocks of protons and neutrons.
Alternatively, you can think of matter like a digital image: If you zoom in enough on an image, you start to see it’s made of individual pixels.
Classical physics governs the movement of things we can see, such as baseballs and planets. Quantum physics is a world we can’t easily see. If any part of quantum is substantially different from classical physics, it is that physics at the quantum scale is not only granular but also “fuzzy.”
When we zoom in on an image, a pixel seems to have a well-defined boundary, or does it? If you were able to zoom in on the atoms and subatomic particles that make up the pixel, you would see that the subatomic particles aren’t well defined. Their boundaries and behavior are somewhat unclear. This is similar to drawing a “perfect” line with a pencil and ruler. If you looked at that line with a microscope, the edges would look more wobbly than straight.
The lack of clarity in quantum mechanics creates unique behaviors. The consequences of these behaviors perplexed the physicists who were the first to try to understand quantum mechanics. These behaviors are:
- Wave-particle duality: Tiny particles look like they are behaving like waves or particles, depending on how you observe them.
- Superposition: In the quantum world, particles can exist in multiple states at once.
- The Heisenberg uncertainty principle: Nature imposes a fundamental limit on how precisely you can measure something. (You can’t measure certain pairs of properties at the same time with unlimited precision.)
- Entanglement: Two things can be so interconnected that they influence each other, regardless of distance apart.
- Spin: Spin is a fundamental characteristic of elementary particles. Like mass or charge, spin determines a particle’s behavior and interaction with other particles.
I will discuss how these behaviors are central to emerging quantum technologies like quantum computing and quantum cryptography and how they manifest in fantastic ways in the natural world.
Wave-Particle Duality
The fuzziness at the granular level occurs because these tiny particles act a bit like waves (similar to water waves and radio waves). Remember the definition of wave-particle duality: Tiny particles like electrons and photons can appear to behave like waves or particles, depending on how you observe them. The wave-like properties of particles at the quantum level are like water waves; they can interfere with one another, resulting in “ripples.” The ripples allow us to predict the particles’ behavior (where they are most likely to be found, what energy they are likely to have and how they will interact with other particles).
Take light as an example.
When light passes through water droplets, the light can act like waves that form the beautiful patterns of a rainbow.
On the other hand, when light hits a solar panel, it acts like a particle. Because we observe the photons’ energy being deposited in chunks (like a solid ball hitting a screen), we perceive them as behaving like particles.
Superposition
To better understand the energetic states of particles, I can draw an analogy to musical instruments. Instruments have many notes (tones, vibrations or frequencies) that they can sound on. When you add energy to an atom, for example, you can excite the cloud of electrons that surround the atom, like striking a drum. Just as a musical instrument can sound on multiple tones because of the mechanical structure of the drum, superposition allows particles to exist in multiple “states” at the same time. This is because of the force or “tension” the nucleus creates on the electron cloud.
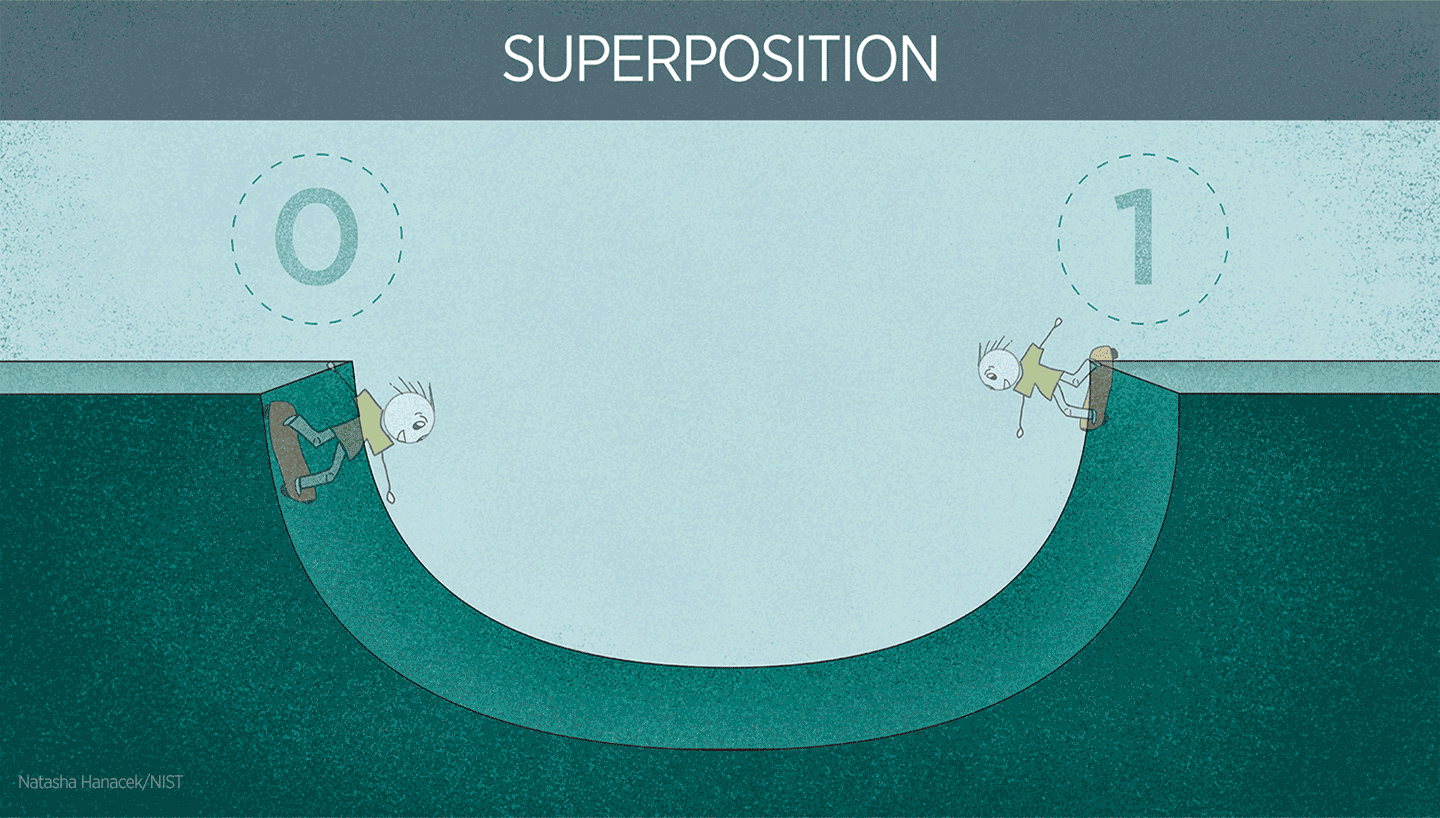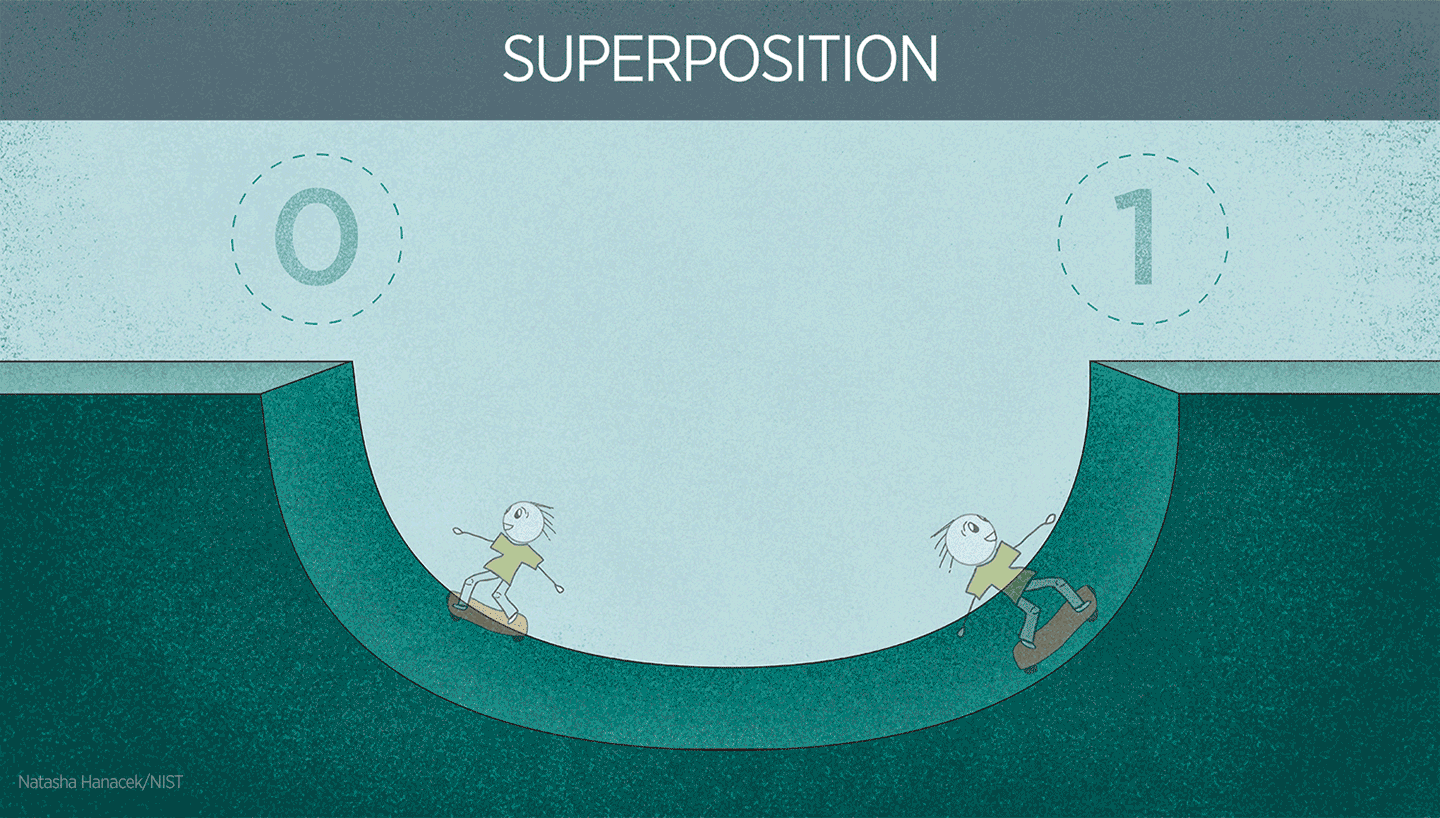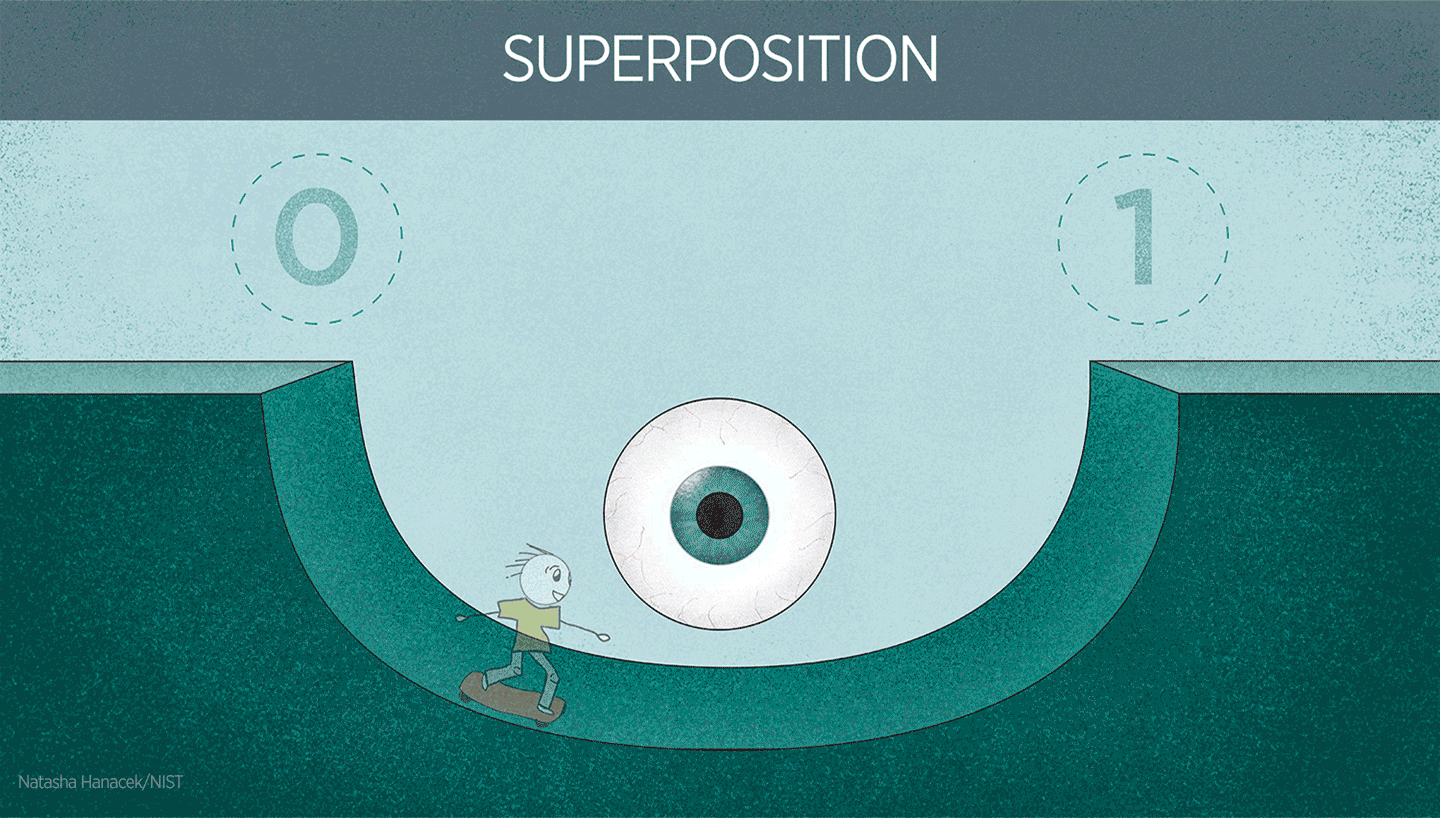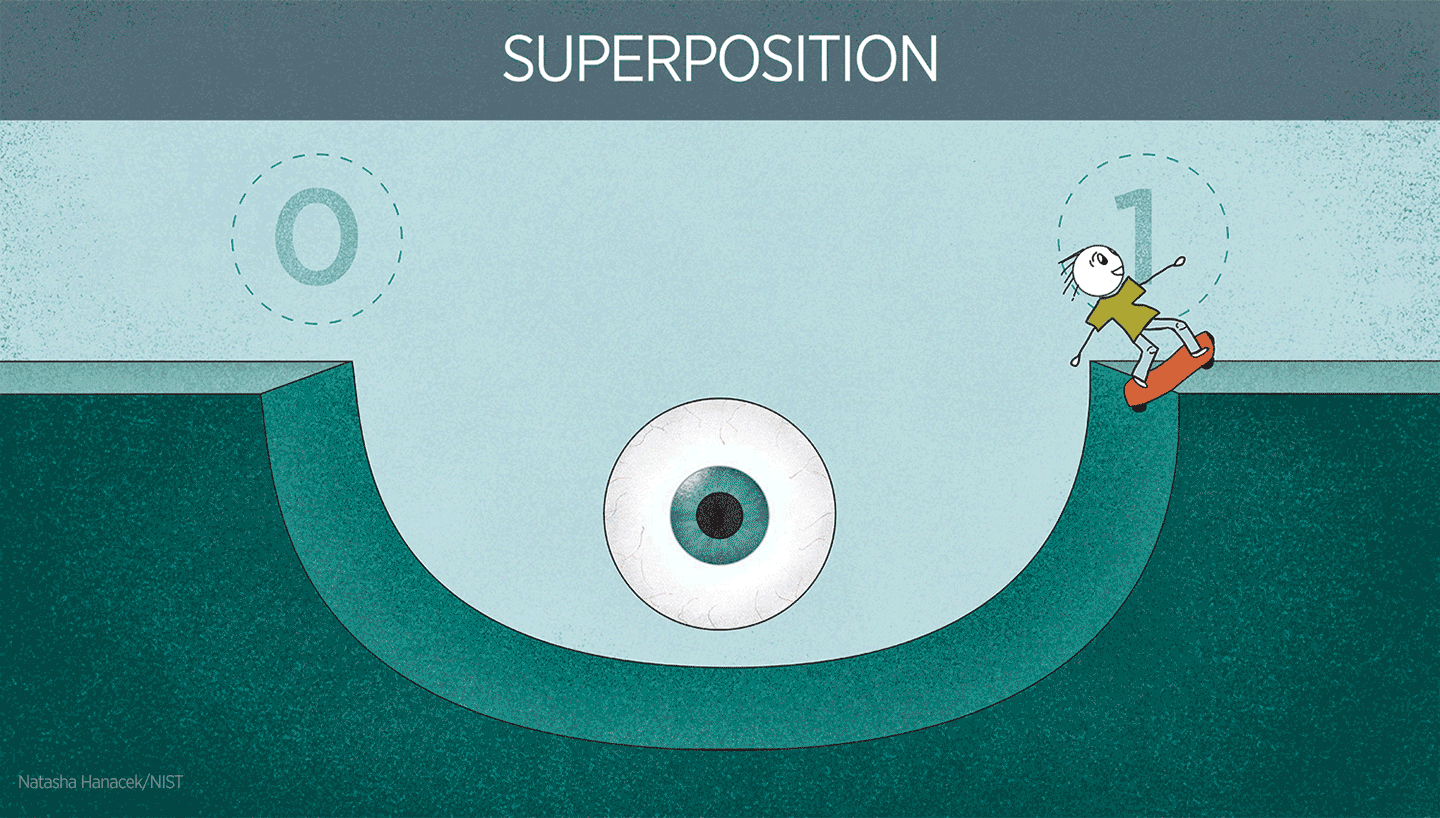
Superposition in action
Superposition is extremely useful in quantum technologies. For instance, superposition is used to make atoms oscillate in atomic clocks. It’s also important to note that physicists have quite a bit of control over superposition in well-controlled systems like atomic clocks. Physicists can control the atom to be in one electronic state or another. Or they can create a superposition of both states.
You can imagine superposition as being similar to a pendulum swinging between positions (one at the far left and one at the far right). When oscillating, the pendulum is at neither position but oscillating from one position to the other. The “swinging” back and forth between the platforms is the oscillation that forms the clock signal, just like the oscillation of a pendulum, just way faster!
Heisenberg Uncertainty Principle in Measurement
The notion of uncertainty exists for measurements of all physical systems but becomes really apparent at the quantum scale.
When you try to measure the state of any system, you inevitably disturb it at some level. Why? Because to observe it, you typically need to interact with it using some type of probe.
For instance, we use photons bouncing off objects to see them with our eyes, a form of measurement that allows us to judge an object’s position, movement and size. The light bouncing off a skyscraper doesn’t have large enough energy to significantly disturb the skyscraper. But if the skyscraper were as small as an electron, the energy could become comparable enough to the skyscraper’s to significantly disturb its state.
This is part of the essence of the Heisenberg uncertainty principle, which says that the act of measurement disturbs the quantum state of the object. As a result, there are limits to how precisely certain pairs of properties, like position and momentum and time and energy, can be known simultaneously.
Entanglement
Quantum entanglement occurs when the quantum states of two or more particles become strongly correlated. This means the state of one particle can instantaneously influence the state of the other, regardless of distance. A common analogy to understand correlation is to think of two entangled photons as two coins that always land the same way when you flip them.

In quantum key distribution (QKD), entangled photons are used to securely exchange cryptographic keys (like in financial transactions for banks or top-secret military messages). If an eavesdropper tries to intercept the photons, the act of measuring them disturbs their quantum state, causing a detectable change in the correlation between the photons. This disturbance alerts the communicating parties to the presence of an eavesdropper, ensuring the security of the key exchange.
Entanglement in action: quantum communication and computation
Entanglement and superposition are used in many of the newer quantum technologies being developed today, such as quantum networking, quantum communication and quantum computing. Quantum bits, or qubits, that are entangled with each other have a potential “quantum advantage” that can allow them to solve some calculations much faster than classical computers and that allows exponential improvement of computing power with the number of qubits.
Spin
While wave-particle duality, superposition, the Heisenberg uncertainty principle and entanglement are all manifestations of the fact that quantum systems have wave-like behavior, spin is off on its own.
Although deeply associated with quantum mechanics, spin is just a characteristic a particle has when it’s created, similar to mass and charge. Despite its name, the term “spin” doesn’t mean the particle is actually spinning.
The spin of electrons, neutrons and protons that make up an atom make it possible for them to form stable structures, such as the elements, planets and our bodies. Your own body and anything you interact with in the physical world exists in its current form because spin gives the particles volume! Electrons can’t occupy the same space because of their given spin. This is what gives matter volume.
Photons have a different spin than electrons, protons and neutrons, allowing them to occupy the same space. This gives photons remarkable qualities. If you have noticed, you can feel the warmth of light, and you can see it, but you can’t hold it or touch it like you can hold things made of matter like pencils, tables and pets.
Spin in action: lasers
The fact that photons can occupy the same space is responsible for the amazing utility of the laser. In lasers, all the photons can perfectly overlap with one another so that all the peaks and troughs of the light waves are perfectly aligned and added together. This allows lasers to create something like a superwave, so all the photons work together in the same space and at the same time. This allows lasers to cut metal, even if they operate with powers similar to a light bulb.
Making Quantum Accessible for All
I am deeply passionate about making quantum mechanics and quantum technology accessible to the public because I envision a future where the applications of these technologies reflect the diverse voices of all demographics.
The impact of quantum technology and computing will be profound. Quantum may bring us more secure communication systems, solve problems like how to design better medicines and much more. It's crucial that everyone has a role in shaping how these innovations evolve to benefit humanity and the planet.
Learn More about the International Year of Quantum!
Visit our International Year of Quantum Science and Technology page to learn about the history of quantum and how it impacts your everyday life.
About the author
Related Posts
Comments
Thank you for this. Very clear and understandable.
As a project manager, I implement hardware and software, then integrate those technologies. By simplifying physics and quantum mechanics misses this point. Quantum technologies are to be used by end users who are not scientists. By focusing on the science, rather than on the application, adoption will be slow. My two cents. Greg Skulmoski
Finally an article written by someone with deep knowledge of a very complex subject because it made sense to an English as foreign language service industry type like me. Thank you.
Isn't entanglement broken by heat? Why is this never mentioned?
Entanglement is a very fragile state that can be broken by many effects, heat being one of them. Heat and other effects that disturb the quantum state are limiting factors in quantum technologies including, atomic clocks and sensors, quantum computers and quantum communications.
This is honestly a top 5 article of all time, thank you sigma <3
Fantastic article, digestible points easily simplifying quantum mechanics! I personally have become so fascinated with everything we are learning with each new discovery! I’m also rather intrigued about the philosophical implications as well! (how can we not be?)
Quantum mechanics challenges our views on reality, determinism, and locality.
Expanding on decoherence, probability, and applications beyond computing could enrich our understanding further. Curious about your take on these philosophical aspects!
Keep up your great work!
Fantastic post! Thank you so much - I now fully get it!
Thanks for a great article. I've always been intrigued by quantum mechanics, and this article had a great description of the phenomena in general terms, while still seeming technically accurate.
A quick question. You mention an example of entanglement being disturbed by a third party observing a photon. However, whenever I've read entanglement described elsewhere, the matching states between particles (which you describe as a correlation) change to match each other instantaneously. Usually, other articles point out that this means information can travel at beyond the speed of light, which would seem to contradict relativity theory. In the example you gave, how does the state of the photon traveling relate to the state of the two entangled particles?
<< If any part of quantum is substantially different from classical physics, it is that physics at the quantum scale is not only granular but also “fuzzy.” >>
Schrödinger, in his famous "cat paradox" paper, was among the first to point out that QP should not be thought of that way (https://en.wikipedia.org/wiki/Schr%C3%B6dinger%27s_cat#Thought_experime…). It's an idea from the very earliest days of QM when he and others were trying to interpret the new theory and constructed the long obsolete "semi-classical" interpretations (described in Max Jammers book). QT does point to an intrinsically probabilistic reality but that's conceptually a very different thing than a fuzzy reality and modern foundations researchers have worked hard to identify exactly where quantum physics really does differ substantially from classical physics (https://arxiv.org/abs/1501.03202).
Likewise, "wave-particle duality", also known as "wave-particle complementarity, is a long obsolete idea; superposition doesn't mean "ontic and"; the HUP is (firstly) about the intrinsic uncertainty in quantum systems rather than about uncertainty caused by measurement disturbance (https://arxiv.org/abs/1904.06139); and other than in "hidden variable" alternative theories to QM, entanglement doesn't entail instantaneous influence (not even the non-signaling kind).
Classical analogies and "semi-classical" thinking too often lead to error, to misconceptions and to misunderstanding QP rather than to understanding it but, somewhat surprisingly perhaps, advances in our knowledge of its conceptual and mathematical foundations have led to a way to explain it that's both fairly easy to understand and correct.
Thanks for your insights! My article was written for a general audience at about a 9th-grade reading level, so the goal was to introduce key ideas in an accessible way rather than dive into technical details or modern interpretations. While I agree that classical analogies can sometimes lead to misconceptions, they also provide useful entry points for younger learners beginning to explore quantum mechanics.
To your specific comments:
• On “fuzziness”: I used the term fuzzy to convey the inherent uncertainty in quantum systems due to their probabilistic nature—whether in internal energy states (like spin or atomic energy levels) or external states (like position and momentum).
• On wave-particle duality: I agree that a more precise term is wave-particle complementarity. For a general audience, I frame it like this: Quantum systems always have wavelike properties—this isn’t something that switches on and off. If you zoom into an electron or photon, they aren’t just tiny hard balls; they are energy. Sometimes, modeling an electron as a particle is useful, and other times, thinking of it as a wave (a wavefunction) is more convenient. The “duality” lies in how we model the system, not in the particle itself.
• On classical analogies and semi-classical thinking: I completely agree that classical analogies can sometimes hinder understanding rather than help. I would have loved to include a deeper discussion of probability and wavefunctions! However, given the target audience, it was decided that a more technical discussion wouldn’t resonate. That said, I believe these ideas are accessible to those with a 12th-grade education and beyond—it’s just difficult to find venues willing to target that level.
• On Heisenberg uncertainty: The simplest way to clarify this is that measurement itself—by requiring interaction between a quantum state and another particle—disturbs the system’s delicate wavelike nature, adding uncertainty to the result. A practical example from atomic clock research is the ongoing effort to develop non-destructive ways to measure atomic states, improving clock precision:
R. Hobson et al., "Cavity-enhanced non-destructive detection of atoms for an optical lattice clock," Opt. Exp. 27 (2019).
Thanks for your response! I admit I have no idea what the appropriate approach for younger audiences might be but if the concept of probability isn't beyond them then I'd suggest that they may be in the enviable position of being better prepared to absorb a thoroughly modern perspective on standard QM than someone who's already absorbed some of the obsoletisms and misconceptions that still dominate "traditional" approaches. As might a "quantum innocent" of any age. It's what my last remark was referring to: the idea that because quantum theory (the maths) is a "conceptually very straightforward generalization of classical probability theory" ( https://arxiv.org/abs/1205.3833 ) it should be possible to explain the basic ideas of QM in simple yet entirely misconception-free terms. In particular there should be no need for any misleading talk of waves or wavelike properties, much less "wave-particle complementarity"; an idea of Bohr's which he himself realised was a mistake ( https://plato.stanford.edu/entries/qm-copenhagen/#Comp ).
Terrific primer. Thank you!
Thank you very mucĥ for taking the time to explain in a way that a laymond like me can understand ( as bezt as i can beìng uneducated past high school)its very fascinating the possibi
liies and you expplained things in a way that got me interested in learning more about a subject that most p
eople ĺike me cant quarrelate into their every day
lives l guess thats why people like you( genuises and i say tht because even educated people have a hard time comprehe ding)) will lead the world into the future thank you
I just did a few presentations to elementary kiddos on quantum science, and found bringing-in some of the names of the young, unencumbered scientists, made it a bit more personal, names that help inspire.
APS Quantum To-Go
Prof. Prem raj Pushpakaran writes -- 2025 marks the birth centenary year of Simon van der Meer, and let us celebrate the occasion!!!
Dear Author,
I think your explanation can help many people in understanding the physical interpretation of terms in quantum mechanics as intended by quantum mechanics scientists. It may still not be able to relieve the feelings of many people - who naturally live in innate intuition: as I feel.
I tried to satisfy myself in making a physical interpretation of quantum particles and their properties. My background is aerodynamics, so I took inspiration from elementary particles of aerodynamics, namely a ring-vortex. I started with the speculation that elementary particles of physics can be visualized as ring-vortices. Ring-vortices are rich in properties as many as those possessed by elementary particles of physics. At that point, I hoped to be able to match the properties of ring-vortices with the properties of elementary particles of physics. Then I had to find a fluid to make a ring-vortex model that matches the properties of elementary particles of physics. For this, I had to re-examine the Michelson Morley experiment, the Lorentz transformation, and Einstein's conclusion about the existence of a subtle medium that was then called ether. I also have to convinced myself that Maxwell's equations can be derived from the incompressible Euler equations. And, ... I found a super fine fluid (SFF) whose density order is as large as the density of dark matter in the solar system.
The modeling I made is very interesting, it turns out to be able to explain the properties of material elements in terms of physics, chemistry, strength of materials, nuclear reaction mechanisms. It can also provide an alternative explanation of cosmology, an alternative to the Big Bang theory. More specifically, I found the origin of photon frequencies, and a very strong indication that gravity is one with the other three fundamental forces. In the modeling of the nucleus of atoms of material elements, I saw that each material element has a unique configuration (due to a number of criteria). The properties of physics, chemistry, material strength, thermodynamics, are closely related to the configuration of the arrangement of protons and neutrons in the nucleus. This modeling provides an explanation from a geometric perspective both on elementary particles of physics or on nuclei, atoms, molecules, and bulk materials. What I have described has been written in an Ebook: From Dark Matter to Ordinary Matter (in fluid mechanics way), available at ResearchGate or at Academia.edu.
Please have a look and read it, feel free to contact me if you are interested in discussing it.
Regards
Djoko
as a person returning to a grade 9 reading level, could you explain the composition of a magnetic field travelling between a north pole to a south pole . and method of controlling the shape of that magnetic field.






Simple yet excellent article