When testing a new medicine, researchers must do more than assess how well that drug works. They also have to determine whether the medicine has some negative, unintended consequences.
To do that now, scientists have a couple of choices in pre-clinical studies: They test the drugs in vitro – that is, with laboratory equipment outside the body – and in vivo – that is, in animal models.
But a growing number of researchers are experimenting with a third way, something that’s sort of in between the two. It’s called organ-on-a-chip, and it involves growing real tissue from a human organ on a small structure that mimics what that organ tissue would experience inside a body.
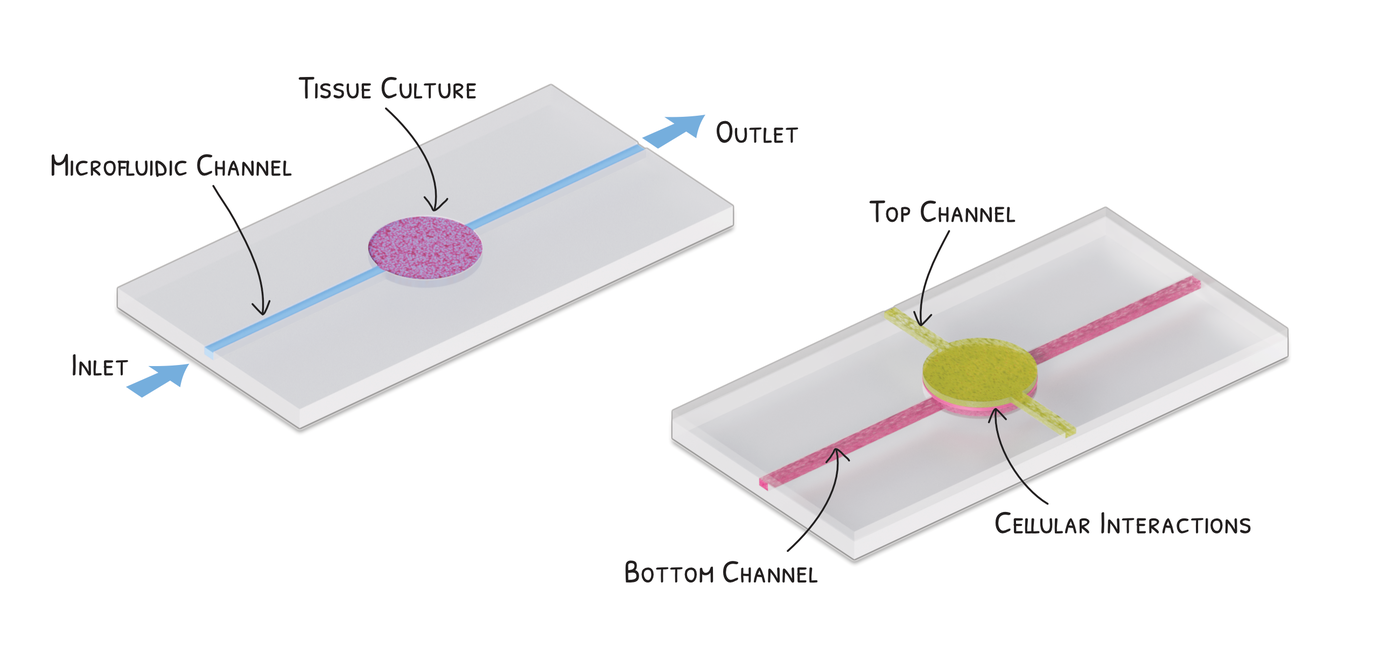
These structures are not computer chips but microfluidic devices – tiny instruments that provide the moist environment that body tissue needs to thrive, complete with a fluid that brings nutrients to mimic, to some extent, what the blood does in the body when flowing past cells. In recent years, more sophisticated microfluidic devices have also been made that expand and contract tissue to simulate, for example, conditions within the lungs.
By mimicking the organ’s microenvironment, researchers hope to get a better idea of how the medications might affect these tissues in a person without relying on an animal model.
Though many groups around the world are developing this technology, each has its own protocols, styles of measurements, and even terminology. As a result, the studies can’t be easily intercompared, which some researchers feel is slowing progress in the field.
To address this need for standardization, NIST is leading a working group to develop a set of guidelines and standards for organ-on-a-chip systems. The group includes researchers from industry, academia, and other government agencies, from around the world.
In a paper published this week in the journal Lab on a Chip the working group laid out their case for why these standards will help the field grow.
“This is step one: to publish a summary of guidelines so that people will start to get interested in standardization,” said NIST’s Darwin R. Reyes, chairperson of the working group. “But the ultimate goal is to get fully developed standards.”
The working group also held a workshop at Michigan State University (MSU) in April 2023. Participants included representatives from industry, academia, and government, including NIST, the U.S. Food and Drug Administration (FDA), and the European Commission, the executive branch of the European Union. NIST co-sponsored the workshop alongside the co-chair of the group, Nureddin Ashammakhi from MSU.
Right now, the group is gathering input from the various organ-on-a-chip stakeholders, people who are researching or funding organ-on-a-chip projects. This involves asking stakeholders what they need, and what recommendations they have for standardizing their own technology. Though there are many organs represented in organ-on-a-chip research, the group has chosen three – the heart, kidney, and liver – as models to begin their data collection.
“The members of the working group want to focus on issues that have to do with the engineering of these systems,” Reyes said. “For example, how would flow actually affect the cells that are in the systems? How do you maintain a number of cells that is in accordance with the ratio of cells that are in real organs? And what volume of fluid in these systems will be comparable to the number of cells we’re putting in to make it similar to real organs?”
One challenge the group faces is that many commercial organ-on-chip devices contain proprietary information, meaning companies making their own devices can’t divulge what’s inside. Any standards the working group develops would focus on the input and the output of the devices, Reyes said, without specifying how the company achieves that result.
NIST is a logical leader for a project like this, Reyes said, because it’s a neutral entity, an institute that doesn’t compete with anyone else.
“Being at NIST allowed me to gather people together from all over the world to tackle this issue of standardizing organ-on-a-chip research,” Reyes said. “They wouldn’t have come in if it wasn’t NIST talking about standards.
“That’s part of our mission, to help industry,” Reyes said. “This is one of the ways we can do that.”
--Reported and written by Jennifer Lauren Lee
Paper: D.R. Reyes, M. Esch, L. Ewart, R. Nasiri, A. Herland, K. Sung, M. Piergiovanni, C. Lucchesi, J.T. Shoemaker, J. Vukasinovic, H. Nakae, J. Hickman, K. Pant, A. Taylor, N. Heinz, N. Ashammakhi. From animal testing to in vitro systems: advancing standardization in microphysiological systems. Lab on a Chip. Published online February 15, 2024. DOI: 10.1039/D3LC00994G

