Context of a Discovery: Dave Wineland
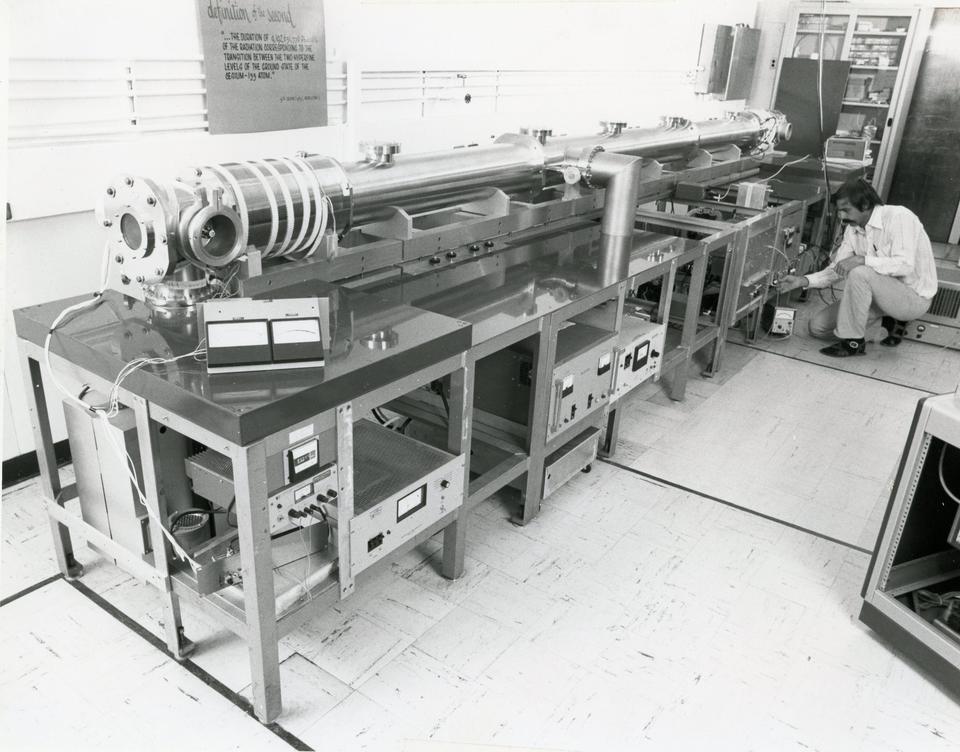
NBS-6, the cesium-atom clock that Wineland helped get up and running, was based on a hyperfine transition at which gaseous cesium atoms absorb and emit microwave radiation with a frequency of slightly more than 9 billion cycles per second, or 9 Gigahertz. After precisely measuring atoms making this transition, physicists operating the clock then broadcast a signal at a frequency precisely related to the transition frequency, which other clocks could then refer to as a sort of “meter stick” for the second. An international body of scientists had chosen in 1967 to use the cesium atom to define the second so that the unit did not depend on anything human-created, but rather on a natural object that, in principle, anybody in the world could access.
To maximize accuracy, David Glaze, who built NBS-6, used a vacuum chamber to isolate the cesium atoms from the environment. But the clock’s accuracy was still limited by the fact that it operated at room temperature, which meant its atoms traveled at speeds averaging around 300 meters per second, close to the speed of sound. Even though these are slow speeds relative to that of light, Einstein’s special theory of relativity predicts this motion to cause the atoms’ oscillations to slow down slightly according to an observer in the lab, an effect called relativistic time dilation.
Scientists had known for some time that atomic clocks would be more accurate if the atoms were cold and the time dilation was small but they had no way to cool individual atoms while keeping them as a gas. Wineland, however, had an idea. Shortly before leaving the University of Washington for NIST, Wineland, along with his mentor Dehmelt, wrote a short paper proposing how to cool trapped ions using laser light. Though they didn’t realize it, Theodor Hänsch and Arthur Schawlow at Stanford University were thinking about the same problem for gases of neutral atoms, and published a paper the same year.
“From today’s point of view, you’d say [the two papers were] the same, but from the point of view of 1975, they looked very different,” says NIST-Gaithersburg Fellow Bill Phillips, who was inspired by them in his quest to laser-cool neutral atoms, for which he would win the 1997 Nobel Prize in Physics.

Wineland’s basic idea was to put ions in a trap and shine a laser beam on them. The ions would absorb the beam's light. The idea takes advantage of the fact that energy transitions of the ions’ electrons make them like sharply tuned radios, capable of absorbing and re-emitting certain frequencies of light but not others. But there was a twist, because an ion moving toward or away from oncoming light sees the light’s frequency shifted, due to the familiar Doppler effect, the same effect that causes a siren’s apparent pitch to change as an ambulance or fire engine drives by. So Wineland’s laser would actually be tuned to a frequency just below that of an energy transition in the ions’ electrons. The trap would be built in a way that the ions would oscillate back and forth, and when they were moving in a direction opposite to that of the laser beam, the frequency of the light would, from the ions’ point of view, appear to Doppler shift to the transition frequency. The ions would absorb the incoming light and re-emit it in all directions. Since photons carry momentum, their absorption would slightly reduce the momentum and velocity of the atoms. The momentum change imparted to the atom from the emitted photons would average to zero, leaving only the laser’s slowing influence.
Wineland had been tasked with helping to make NIST’s cesium clock operational, but his group leader, Helmut Hellwig, was able to get funding from NIST for laser-cooling experiments. Though they would not necessarily deliver an immediate practical payoff, NIST leadership realized they could lay the foundation for much more accurate future clocks.
In 1978, less than a year after they began, Wineland, along with NIST colleagues Robert Drullinger and Fred Walls, laser-cooled a cloud of trapped magnesium ions. They could sense the ions’ velocities by detecting the associated electrical currents induced in the trap electrodes. The researchers were thus able to infer the ions’ temperature, which turned out to be around 40 kelvins (-233 degrees Celsius or -388 degrees Fahrenheit).

Meanwhile, researchers at the University of Heidelberg in Germany, along with Dehmelt, who was visiting at the time, cooled barium ions using the same method. The two groups’ papers arrived at the office of the journal Physical Review Letters within a day of each other. Though the research groups had come nowhere near the coldest temperatures scientists had reached with traditional chilling devices such as helium dilution refrigerators, the two papers marked the world’s first demonstrations that light alone can cool matter.
“The big thrill for us was just to see the effect,” Wineland recalls. “The effect was very clear.”
The early success had a profound effect on Wineland’s career.
“Laser cooling has been a part of nearly every experiment I’ve done since,” he says.
In 1979, Wineland and NIST colleague Wayne Itano wrote a paper explaining the theory of laser cooling. They then set their sights on creating the first laser-cooled atomic clock. They achieved this goal in late 1984, when along with John Bollinger and postdoctoral researcher John Prestage, they laser-cooled beryllium ions to a temperature of around 100 millikelvin (one-tenth of a kelvin) and measured the frequency of an electron transition similar to that used in cesium clocks. The precision of the researchers’ experimental beryllium clock was comparable to that of NBS-6.
Beyond doing practical work developing new clocks, Wineland and his colleagues were, for the first time, gearing up to experimentally implement some of the fanciful ideas that quantum theorists had cooked up more than a half-century earlier. In the 1930s, Austrian-born physicist Erwin Schrödinger wrestled with the question of what it would mean to put an object into a quantum “superposition.” Superposition is a concept fundamental to quantum mechanics, according to which objects, usually at the tiny submicroscopic scale of atoms, can act as if they are in multiple energy states or even different places at the same time. Schrödinger arrived at the seemingly absurd conclusion that if one takes quantum superposition seriously, one has to conclude that even a macroscopic object such as a cat, which is a collection of uncountable billions of billions of atoms and molecules, could be simultaneously dead and alive.
During more than half a century, nobody had figured out how to control systems well enough to test such ideas in the lab on individual quantum objects. But Wineland and his colleagues would soon turn their lasers and ion traps toward doing exactly that.


“Dave is someone who wants to have the science first, and does not look at the competition just as a fierce game. He clearly tries to get the best out of collaboration with colleagues.”
– Serge Haroche, physicist at Collège de France and the École Normale Supérieure in Paris; shared 2012 Nobel Prize in Physics with Dave Wineland

