Summary

NOW AVAILABLE
The NIST monoclonal antibody (NISTmAb) reference material, RM 8671, is intended for use in evaluating the performance of methods for determining physicochemical and biophysical attributes of monoclonal antibodies. It also provides a representative test molecule for development of novel technology for therapeutic protein characterization. The RM is intended for a variety of uses that may include system suitability tests, establishing method or instrument performance and variability, comparing changing analytical test methods, and assisting in method qualification.
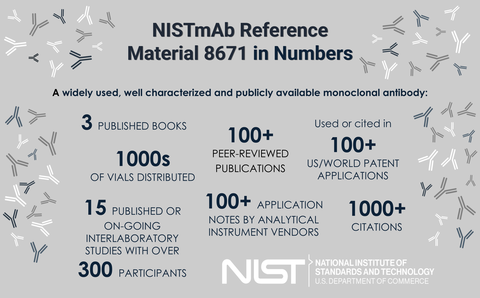
The NIST monoclonal antibody reference material is, quite possibly, the most widely characterized publicly available monoclonal antibody, a molecule directly relevant to the biopharmaceutical industry. It is grounded in quality measurements, thus providing a common control material for originator and follow on manufacturers alike. The voluntary and open access nature of this material makes it the premier choice for technology development in the pre-competitive space.
Background:
Comprehensive analysis of monoclonal antibody therapeutics is no easy task. These molecules embody various complex attributes, the characterization of which is a long and arduous process, yet monoclonal antibody therapeutics have taken residence as perhaps one of the most influential therapeutic classes of our time. An inter-continental crowdsourcing characterization of a single IgG1k (NISTmAb) was recently reported as a three volume book series, serving as a supportive tool in the evolution of analytical and biophysical methodologies. The NISTmAb case study provides a comprehensive overview of monoclonal antibody therapeutics, using the NISTmAb as a vehicle for highlighting the characterization stages of product development. Contributors utilized the NISTmAb throughout, demonstrated the potential utility of class-specific reference materials as a means to facilitate open innovation, and identified a number of emerging research areas for future development. Conclusion of the series is therefore met with eager anticipation of continued biopharmaceutical advancement through industry-focused partnerships.
Qualification, certification, and lifecycle management of the NISTmAb reference material 8671, to be publicly released in 2016, will be a representative means by which this collaboration will continue. In preparation of the material for public availability, many methods were qualified for their intended use in assessing the identity (e.g., peptide mapping), purity (e.g., capillary zone electrophoresis [CZE]), monomeric purity (size exclusion chromatography [SEC] and capillary sodium dodecylsulfate electrophoresis [CE-SDS]), and stability (dependent on attributes) of the NISTmAb. Additional characterization assays of dynamic light scattering and flow imaging analysis of protein particulates were also employed. Forced degradation studies were performed in order to further elucidate potential degradation pathways and production of product-related impurities relevant for challenging methods during qualification exercises. Accelerated stability studies were also performed to identify adequate storage and handling criteria appropriate to the materials intended use.
Development of innovative technology at NIST and in collaboration with industry stakeholders has also continued. Three interlaboratory studies have been initiated using the NISTmab to evaluate the precision of hydrogen deuterium exchange mass spectrometry, nuclear magnetic resonance spectrometry, and glycoanalysis. The NISTmAb is also serving as the current basis for advancing measurement techniques at NIST such as small angle neutron scattering, nuclear magnetic resonance, x-ray diffraction crystallography, small angle X-ray scattering, mass spectrometry multi-attribute method, and glycan and peptide mass spectral libraries, to name a few.
We hope this material finds widespread utility in the biomanufacturing community. We look forward to industry feedback on the technical utility of NISTmAb RM 8671 as well as the suitability of related follow on materials that may supplement this robust and critical class of therapeutic.
Description
The NISTmAb material is a recombinant humanized IgG1κ expressed in murine suspension culture. It is an »150 kDa homodimer of two identical light chains and two identical heavy chains linked through both inter- and intra-chain disulfide bonds. The protein has low abundance post-translational modifications including methionine oxidation, deamidation, and glycation. The molecule also has N-terminal pyroglutamination, C-terminal lysine clipping, and glycosylation of the heavy chains. These and other product quality attributes were extensively characterized in the ACS book series "State of the Art and Emerging Technologies for Therapeutic Monoclonal Antibody Characterization" for the initial batch of material (interim material 8670), used as the Primary Standard (PS) herein. Multiple bulk substance containers were homogenized to form a second batch (14HB batch) of material that was aliquoted into 1 L containers. A single 1 L container of 14HB was diluted 10-fold and aliquoted as RM 8671 lot 14HB-D-001.
A vial of RM 8671 contains 800 µL of 10 mg/mL IgG1κ monoclonal antibody in 12.5 mmol/L L-histidine, 12.5 mmol/L L-histidine HCl (pH 6.0). The material was produced in murine suspension cell culture and has undergone industry standard upstream and downstream purification to remove process related impurities.
For additional information, please visit the NISTmAb dashboard via the NIST Biomanufacturing Resource Hub (NBRH)

Major Accomplishments
NISTmAb RM 8671 Summary of 5 Year Stability Verification (5YSV)
ACS Book series: "State-of-the-Art and Emerging Technologies for Therapeutic Monoclonal Antibody Characterization"
- Volume 1 - Monoclonal Antibody Therapeutics: Structure, and Regulatory Space
- Volume 2 - Biopharmaceutical Characterization: The NISTmAb Case Study
- Volume 3 - Defining the Next Generation of Analytical and Biophysical Techniques
Analytical and Bioanalytical Chemistry Series
The NISTmAb Reference Material 8671 lifecycle management and quality plan
The NISTmAb Reference Material 8671 value assignment, homogeneity, and stability
Qualification of NISTmAb charge heterogeneity control assays
Development of orthogonal NISTmAb size heterogeneity control methods
Development of an LC-MS/MS peptide mapping protocol for the NISTmAb
Characterization of the NISTmAb Reference Material using small-angle scattering and molecular simulation
Part II: Concentrated protein solutions
The NISTmAb Reference Mass Spectral Libraries and Related Publications
The development of the three NISTmAb mass reference spectral libraries provides comprehensive data of tryptic peptides and their various biological modifications required to support industry’s need in determining the properties of mAbs with high-degree heterogeneity. All identified peptides produced in the tryptic digests of a humanized IgG1κ reference material (NISTmAb) are selected from over six million peptide-spectrum matches acquired by high-resolution, accurate-mass 1D/2D LC-MS/MS analyses. These cover 99% of the NISTmAb sequence, representing 211 of 213 light chain residues and 444 of 450 heavy chain residues. A highly complex glycosylation profile was achieved for the NISTmAb, including 60 unique glycan compositions, almost more than double earlier reports for any individual mAbs expressed in CHO, NS0, and other cells. A complete glycation profile was determined, for the first time, for all possible glycation sites in the NISTmAb. A spectral library-based novel workflow for complete disulfide mapping of the nine NISTmAb native SS bonds as well as 86 SS bonds arising from experiment artifacts. Fragments from various peptide, glycopeptides, and disulfide-linked peptides in all three libraries are fully annotated.
NISTmAb Mass Spectral Library of Human IgG1κ mAb Drugs
- 3,360 peptides
- 12,608 spectra
- Extensive degradation, glycation, oxidation, and cysteine variation
- Over 20 types of analytical artifacts
- Based on 1D/2D LC-MS studies
- Multiple HCD collisional energies
Glycopeptide Spectral Library
- 1,703 spectra
- 247 multiply charged glycopeptides
- 81 different N-glycans (NS0 and CHO)
- 20 and 24-fraction 2D-LC studies
- Energy-dependent changes in HCD fragmentation of glycoforms
Disulfide-Linked (SS) Peptides Spectral Library
- 702 consensus mass spectra of SS linked peptides
- 596 selected “best” reference spectra of SS linked peptides
- 155 different peptides arising from SS linkages in NISTmAb
- 207 different peptides from scrambled SS linkages
References
- Qian Dong, Xinjian Yan, Yuxue Liang, and Stephen E. Stein, J. Proteome Res. 2016, 15, 5, 1472-1486
- Qian Dong, Yuxue Liang, Xinjian Yan, Sanford P. Markey, Yuri A. Mirokhin, Dmitrii V. Tchekhovskoi, Tallat H. Bukhari & Stephen E. Stein mAbs, 2018, 10:3, 354-369
- Qian Dong, Xinjian Yan, Yuxue Liang, Sanford P. Markey, Sergey L. Sheetlin, Concepcion A. Remoroza, William E. Wallace, and Stephen E. Stein, Proteome Res. 2021, 20, 3, 1612–1629
Library Download
https://chemdata.nist.gov/dokuwiki/doku.php?id=peptidew:mab
NISTmAb Interlaboratory Study on Glycosylation Analysis
In 2020, an interlaboratory study of glycosylation profiles of a reference and modified IgG antibody involving 103 reports from 76 laboratories was reported by Stephen Stein and Lorna A De Leoz et al., in Mol Cell Proteomics. 2020 Jan;19(1):11-30.
Highlights
- An interlaboratory study of the glycosylation of a reference antibody: NISTmAb.
- 103 reports were received from 76 diverse laboratories worldwide.
- Analysis involved two samples, the NISTmAb and an enzymatically modified sample, enabling within-lab separation of random and systematic errors using the “Youden two-sample” method.
- Consensus values were derived and similar performance across all experimental methods was noted.
NMR Round Robin
Critical quality attributes (CQA) are significant measurement parameters of a medical product that impact both product safety and efficacy and are essential characteristics that are linked to positive public health outcomes. One CQA, higher order structure, is directly coupled to the function of protein biologics (biopharmaceuticals), and deviations in this CQA may be linked to pathological functions (e.g., immunogenicity or toxicity). NMR can yield structural fingerprints for a protein biologic at atomic resolution that are intrinsically dependent on higher order structure. While NMR spectral methods are well established for small molecules, peptides and small proteins, these approaches are far from standard or routine for proteins above 30 kDa in size, such as monoclonal antibodies (mAbs).
The primary goal of the NMR interlaboratory project is to use the Fab domain from the NISTmAb to demonstrate the robustness of the NMR measurement and to validate NMR structural fingerprinting measurements for the assessment of higher order structure of large protein biologics and/or domains from these proteins. The validation of NMR methods for the characterization of the higher order structure of mAbs is specifically targeted due to the large interest of the pharmaceutical industry in using mAbs as platforms for therapeutic development. The project involves a total of 30 partners in 10 countries, including Canada, United States, United Kingdom, Sweden, Switzerland, Germany, Slovenia, Brazil, Australia, and Japan. Results will be published in a peer reviewed journal.

