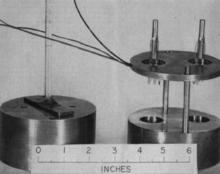
1950s: Calorimetric comparisons of national Ra standards
In the early 1950s, Wilfrid Mann worked with collaborators in Canada, United Kingdom and Germany to organize bilateral intercomparisons of the national standards (Hönigschmid standards) of the three countries and the United States. Most of these comparisons were done using electroscopes or other means of gamma-ray measurements. Hönigschmid had taken great care in preparing the 1937 standards and all of the exhaustive comparisons mainly served to verify his stated mass values. The only new method to be applied was a microcalorimetric technique developed by Mann. His radiation balance was a twin-cup Peltier-type microcalorimeter. The cups of the balance were of gold, and the balance was enclosed in a temperature-attenuating enclosure, which gave a precision of about ± 0.1 % in comparing the Hönigschmid standards.
The advantage of the microcalorimeter was because response depended on the power generated by the radium-226 decay, eliminating the uncertainties in the gamma-ray measurements introduced by source self-absorption and variable thicknesses in the glass walls. It was necessary, however, to account for the Radium-D (lead-210) and progeny ingrowth from 1934. Making all the appropriate corrections, and using Hönigschmid's mass values, Mann arrived at an average power per unit mass of 165.83 µW/mg of radium element. At the conclusion of these intercomparisons, Mann and his contemporaries agreed that the set of Hönigschmid standards constituted the international standard for radium-226.
By the end of the 1950s, radium-226 standards were no longer considered separate from other standards of radioactivity. The International Radium Standards Commission had evolved into an International Committee for Radiation Quantities and Units. The task of developing and maintaining international standards of radioactivity now fell to Section II of the Consultative Committee for Ionizing Radiation Measurements.
Created August 13, 2009, Updated September 26, 2016