Summary
We provide SI-traceable reference materials for use by calibrant and reagent manufacturers in production of their own calibrants and standards. Clinical laboratories with in-house methods can use the reference materials for production of their own quality control materials, which will be traceable to a NIST standard.
BK Virus DNA Quantitative Standard, NIST SRM 2365
JC Virus DNA Quantitative Standard, NIST SRM 2367
Description
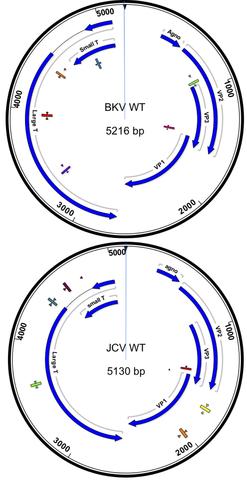
Whole BKV or JCV virus genomes have been inserted into a cloning vector, propagated in Escherichia coli, extracted, linearized, and certified for number of copies of the genome per volume (e.g. copies per microliter). The DNA was sequenced and provided as information value in the certificate of analysis.
BK virus (BKV), originally described in 1971 [1], is a 5kb polyomavirus which shares roughly 75% of its DNA sequence with JC virus (JCV). Nearly 80% of the population is seropositive for these viruses [2, 5-6]. Although BKV disseminates into the urinary tract and kidneys, it usually remains latent [2]. However, immunocompromised patients, such as those undergoing kidney or multiple organ transplants [3], severe reactions as a result of BKV infection may occur. The infection presents itself in the clinic as renal dysfunction and abnormal urinalysis. If the BK virus replicates within the new graft, BK-associated nephropathy (BKVAN) may arise [4]. BKVAN is thought to occur as a result of high doses of immunosuppressants administered to transplant patients that also have BKV infections (1-10% of kidney transplant patients). The devastating consequence of BKVAN is graft rejection, which occurs in up to 80% of infected patients. Therefore, in order to determine an optimal immunosuppressant dosing regimen, accurate measurement of BK viral load in transplant patients is needed.
JCV also arises in immunocompromised patients: AIDS, transplant, those undergoing treatment for cancer, MS, psoriasis, or those with Hodgkin's Lymphoma. JCV can cause progressive multifocal leukoencephalopathy (PML), a progressive, demyelinating disease that often becomes fatal. Currently, there is no therapy for JCV and labs monitor viral load and adjust immunosuppressant dose.
DNA reference materials for these viruses allow accurate measurements of viral load and enable commutability of measurements among laboratories across the world.
[1] Gardner SD, et al, 1971. "New human papovavirus (B.K.) isolated from urine after renal transplantation". Lancet. 1 (7712): 1253-7.
[2] Egli A, et al, 2009. "Prevalence of Polyomavirus BK and JC Infection and Replication in 400 Healthy Blood Donors". Journal of Infectious Disease. 199: 837-46.
[3] Gupta, G, et al, 2006. "Low Incidence of BK Virus nephropathy after simultaneous kidney pancreas transplantation". Transplantation. 82 (3): 382-8.
[4] Fishman, JA, 2002. "BK Virus Nephropathy - Polyomavirus Adding to Injury". New England Journal of Medicine. 347 (7) 527-530.
[5] Kean, Jaime M., et. al, 2009. "Seroepidemiology of Human Polyomaviruses". PLoS Pathogens. 5 (3): e1000363.
[6] Egli, Adrian, et. al, 2009. "Prevalence of Polyomavirus BK and JC Infection and Replication in 400 Healthy Blood Donors". The Journal of Infectious Diseases. 199 (6): 837–46.
Major Accomplishments
For BKV:
- The BKV wildtype genome (Accession No. JQ713822.1) was synthesized, ligated into the pUC57-AMP plasmid, and transformed in to Sure2 E. coli cells by Genewiz.
- Bulk BKV DNA was gigaprepped and linearized at NIST.
- Multiple primer and probe sets were optimized for digital PCR at NIST.
For JCV:
- The JCV wildtype genome (Accession No. J02226.1) was synthesized, ligated into the pUC57-AMP plasmid, and transformed in to Sure2 E. coli cells by Genewiz.
- Bulk JCV DNA was gigaprepped by Genewiz.
- Bulk JCV DNA was linearized at NIST.
- Multiple primer and probe sets were optimized for digital PCR at NIST.

