Comparing the Biological Responses of Photoluminescent Silicon Nanoparticles with Silicon Micron-sized Particles
Summary
Photoluminescent silicon nanoparticles have a bright and stable fluorescence and are promising candidates for use in bio-imaging, cell staining and drug delivery. In collaboration with the FDA and University of Maryland scientists, the biocompatibility of photoluminescent silicon nanoparticles (3.0 ± 0.1 nm) and silicon microparticles (100~3000 nm) was tested with murine macrophage cell line RAW 264.7 using standard protocols for micron sized particles to measure cytotoxicity and inflammatory responses.
Description

Intended Impact
Technology is now available to produce nanoparticles of uniform size and shape which may allow their use in a wide range of applications from drug delivery to water purification. The high surface area to volume ratio of nanoparticles makes them particularly good catalysts as well as increasing their absorption to biological molecules. The size and surface charge of nanoparticles enable them to access cellular membranes, where larger particles may be blocked. Production and use of nanoparticles less than 100 nm in diameter may result in unknown risks since the exposure of biological systems to novel materials of this size has not been adequately studied.
There are many healthcare products currently available that incorporate nanoparticles, all of which the FDA must regulate and decide if they are safe and effective for their intended use. Few systematic studies dealing with both cytotoxicity and inflammatory responses of cells treated with nanoparticles have been done. How will a biological system react when exposed to nanoparticles? What is the fate of the nanoparticles once they are exposed to a population of cells? If the nanoparticles enter the cell, what effects will they have internally? If nanoparticles are to be used in a biomedical application these questions must be answered to ensure patient safety.
ASTM has developed the first standard test method for biological responses specific to nanoparticles to particles in vitro, using murine macrophages, cells important in the inflammatory process. However, it is not clear whether many common toxicity protocols written for micrometer-size particles are applicable to nano-size based biomaterials and products. This test is an in vitro test that uses murine macrophages, call that are important in the inflammatory process.
Objective
Since the use of 1-100 nm nanoparticles may result in unknown biological risks and no clear confirmation exists to show that established standard test protocols for larger particles are appropriate, it is important to develop reliable methods to study the potential cytotoxicity and inflammatory response of nano-sized particles in vitro and in vivo. The present study of murine macrophages exposed to silicon nanoparticles, will help define safety requirements for biomedical applications of nanoparticle.
Goals
- Determine the biocompatibility of photoluminescent silicon nanoparticles using standard protocols to measure cytotoxicity and inflammatory responses for micron sized particles with murine macrophage cell line RAW 264.7.
- Compare the biological responses between silicon nanoparticles (3.0 ± 0.1 nm) and silicon microparticles (100~3000 nm) in murine macrophage cells.
Research Activities and Technical Approach
To investigate the biological responses of silicon nanoparticles (3.0 ± 0.1 nm) and silicon microparticles (100~3000 nm) in murine macrophage cells, the silicon nanoparticles and microparticles were dry heat sterilized to ensure they were free of endotoxins. Particles with and without lipopolysaccharide (LPS) were incubated in macrophage cells. Cells were examined for gross morphological appearance by phase contrast microscopy, as shown in the figure. Cytotoxicity was detected by trypan blue dye exclusion and MTT assays, and the cell supernatants assayed for production of tumor necrosis factor-alpha (TNF-alpha), IL-6 and nitric oxide.
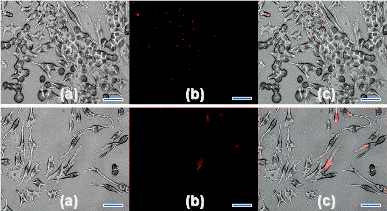
Figure 1. Phase (i), fluorescence (ii), and combined (iii), images of RAW 264.7 macrophages incubated (24 h) with red fluorescent (λem. 640 nm) silicon nanoparticles (<4 nm in Dia.). A: Control (RAW 264.7), B: SNs concentration of 20 µg/ml, C: SNs concentration of 50 µg/ml, scale bar: 30 μm.
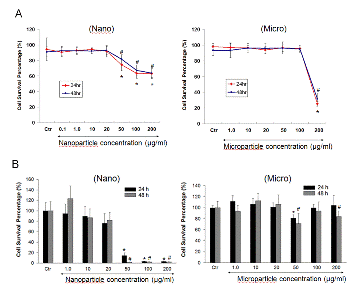
Figure 2. Effect of SNs and SMs on cell survival percentage in RAW 264.7 cells based on trypan blue dye exclusion (A) and MTT (B) assay. Cells were treated with different concentrations (0.1~200 μg/ml) of SNs and SMs for 24 and 48 h. At the end of treatment, trypan blue stain was added to a oliquot of cells to assess the cell live/dead ratio (A). Yellow MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole) was introduced into the 96 wells containing cells incubated for 24h with either nano or microparticles. Live cells convert the yellow colored dye to purple color which is then measured with a fluorescence plate reader. * and # indicate a statistical difference from the control, p<0.05.
Major Accomplishments
- No statistically significant cytotoxicity or inflammatory responses were detected with silicon nanoparticles at concentrations up to 20 μg/ml.
- Concentrations of nano and micro particles higher than 20 and 200 μg/ml, respectively, with and without addition of LPS showed a statistically significant increase in cytotoxicity.
- Si nanoparticles stimulated macrophages to down-regulate cytokines (TNF-a, and IL-6). It is conceivable that macrophages could not identify the nanoparticles as "foreign materials".
- Fluorescence microscopy showed that Si nanoparticles could enter macrophages, however because of their small size or atypical method of entry into the macrophage, nanoparticles are not recognized as "foreign" by the macrophages. Silicon nanoparticles entered the macrophage cell through the pores in the cell membrane, while the microparticles are large enough to be recognized as "foreign" and were phagocytized.
- Particle size was observed to be a decisive factor in determining cytotoxicity as other known factors were eliminated. The higher Si nanoparticle cytotoxicity at equivalent gram concentrations may be a result of higher molar concentration and relative surface area as well as the enhanced intracellular access.
Associated Publications:
Choi, J. et al. Comparison of cytotoxic and inflammatory responses of photoluminescent silicon nanoparticles with silicon micron-sized particles in RAW 264.7 macrophages. Journal of Applied Toxicology 2008, 29(1), 52-60.

