Summary
Discovery of novel materials to facilitate hydrogen gas production has prompted new avenues of research towards developing hydrogen as an alternative to fossil fuel. Optimizing candidate material properties for improved hydrogen generation per unit weight, rapid kinetics, and high turnover in synthetic catalytic systems are still difficult to attain. To aid in this research, we employ ultrafast time-resolved infrared (TRIR) spectroscopy as a measurement tool to better understand the intrinsic vibrational modes and complex molecular photodynamics of di-iron hydrogenase mimics (see Figure below) of a bacterial light-driven hydrogen-producing biocatalyst. These measurement modalities permit direct evaluation of systems capable of meeting efficient hydrogen production levels. In this work, TRIR and FTIR spectroscopies are applied to measure internal vibrational energy reorganization dynamics and identify specific vibrational modes of model species for the reactive center of the hydrogenase protein in active bacteria. Through a long-standing collaboration with Prof. Christopher Stromberg and his students from the Department of Chemistry, Hood College, various CO- and CN-derivatized versions of the Fe-Fe hydrogenase active site have been investigated using these measurement tools. Our measurements have identified specific vibrational modes in the mid-to-far (THz) infrared spectral regions for the different isomeric forms of these mimics. Time-resolved IR measurements, employing UV and visible photolysis, reveals different dynamics including CO-ejection and Fe-Fe bond extension occurs. Current studies include measurement of specifically substituted Trimethylphosphine and CN-containing homologs to better understand hydrogen generation reaction yields and transient species.
In collaboration with Prof. Edwin Webster and students at the University of Memphis, DFT methods are employed to help with mode assignments, electronic state and reaction energetics.
Description
Time-resolved infrared (TRIR) measurements on the picosecond to microsecond timescales and FTIR transmission spectroscopy in the far-infrared (THz) spectral region are applied as a means to monitor light-initiated vibrational mode dynamics in model hydrogenase species. Synthesis of the model species is conducted at Hood College using literature methods while THz FTIR spectroscopy and TRIR measurements are conducted at NIST. Theoretical modeling of Raman, IR and transient species' vibrational modes is conducted at Hood, NIST, and the University of Memphis, using Gaussian 03 Density Functional Theory. To date, detailed vibrational mode assignments for the Fe-Fe(CO)6 and trimethylphosphine derivatives of the ethyl-bridged and propyl-bridged (see Figure b) and c) below) isomeric species have been performed and published. Studies of these same samples under UV and visible excitation conditions have helped identify transiently vibrationally hot species, CO-loss products and electronically excited states that extend the Fe-Fe bond distance to create strongly absorbing transient state in room temperature n-hexane and acetonitrile solutions.
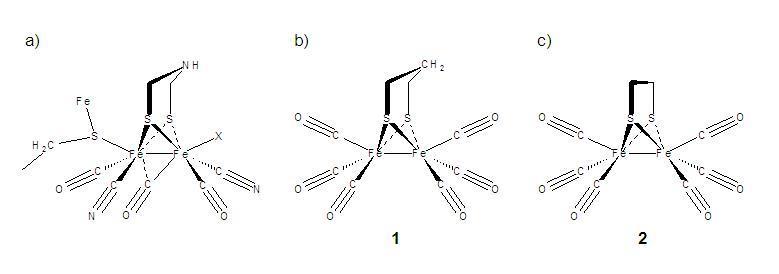
Molecular structures and mimics for the active site of Di-Iron Hydrogenase. a) active site of bacterial protein connected through an Fe-S linkage, b) hexacarbonyl mimic with a propyl disulphide bridge and c) same as b) with an ethyl bridge. Related CN- and PMe3 substituted compounds are also under investigation.
Examples of TRIR spectra in the CO-stretching region (near 5 microns wavelengths) are shown in the Figure below. Evolution of transient IR spectra taken several picoseconds after excitation through 750 ps time delays are presented as waterfall plots (green to blue, respectively).
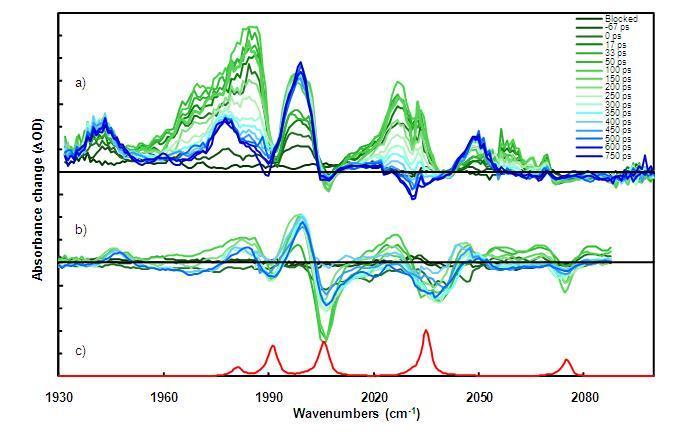
TRIR spectra for the propyl-bridged Fe-Fe(CO)6-Hydrogenase model compound in n-hexane at 293 K using a) 532 nm pump, b) 355 nm pump, IR probe spectra, and c) steady-state FT-IR spectrum of the compound for reference.
Major Accomplishments
- Applied THz FTIR methods to measure solution-phase and solid-state spectra of Fe-Fe hydrogenase model compounds.
- Using Gaussian 03 DFT spectral modeling code, assignments for two CO-containing ethyl and propyl-bridged compounds were made and published.
- Acquired time-resolved infrared spectra from picosecond to microsecond timescales for both ethyl and propyl-bridged hexacarbonyl, cyano and trimethyphosphine species. Used 266 nm, 355 nm and 532 nm excitation wavelengths to resolve different reaction and energy transfer dynamics.
- Application of different excitation energies revealed CO-loss and excited state molecular distortions occur in these systems. Transient extension of the Fe-Fe bond length enhances CO-stretch absorption intensity but does not break the bond. Publications cited below.

