Protocol for Preparing Fibrillar Collagen Matrices on Untreated Polystyrene Labware (Petri)*: A Potential Reference Extracellular Matrix With Robust and Reproducible Cell Responses
Summary
Background:
Collagen type 1 is the most prevalent extracellular protein in connective tissue, and is part of the family of collagens that are fibril forming. Neutralizing monomeric collagen (tropocollagen) solutions that are stabilized in acidic conditions (typically in HCl or acetic acid) will cause the tropocollagen to self-assemble into supramolecular fibril structures, where fibrillogenesis is enthalpy driven. Placing hydrophobic substrates in a freshly neutralized collagen solution reproducibly results in the creation of an adsorbed collagen fibril matrix that is highly homogenous and robust. These matrices have a number of desirable qualities, which include: (i) minimal light scattering in comparison to collagen gels, (ii) mechanical and chemical properties that can be modulated, and (iii) a supramolecular structure physiologically relevant to the configuration of collagen in vivo. Aside from the work our group as presented using these model matrices (see literature list below), others employed these matrices in applications of studying cell (2) and von Willebrand factor-platelet adhesion (3), and in stem cell engineering (4).
Supplies required:†
1. Purified bovine collagen monomer solubilized and stabilized in an acidic solution, stock concentration: typically ~3 mg/ml. After obtaining always store at 4 °C and keep sterile.
Note: to our knowledge, the source of the collagen (e.g., bovine, mouse, human, etc.) does not matter for making collagen fibrils matrices. However, we have found collagen sterilized by gamma irradiation or solvents will not form collagen fibrils or collagen gels. This is likely due to denaturation of the protein structure that has been reported in the literature.
Product such as: PureCol®, Advanced BioMatrix, Phone: (800) 883-8220, www.advancedbiomatrix.com2. NaOH (0.1 M in water): filter sterile, 4 °C.
Product such as: Sodium Hydroxide Pellets, Semiconductor Grade 99.99%, Sigma Aldrich
Filter 0.1 M solution through sterile culture flask such as Millipore Filter Flask with 0.2 µm filter
3. DPBS (10x): premixed, filter sterile. After obtaining always store at 4 °C and keep sterile.
Product such as: Dulbecco's Phosphate Buffered Saline (10x), Invitrogen
4. DPBS (1x): premixed, filter sterile. After obtaining always store at 4 °C and keep sterile.
Product such as: Dulbecco's Phosphate Buffered Saline (1x), Invitrogen
5. Untreated polystyrene Petri dishes or multi-well plates.
Product such as: BD Falcon, Nunc. Make certain these are untreated polystyrene.
Fibrils will not form on plasma treated polystyrene surfaces.
6. 2 Teflon squirt bottles
Product obtained from supplier such as Fisher Scientific
Normal LDPE squirt bottles should not be used
7. Dry, filtered nitrogen source
Such as a nitrogen gun that hooks up to a nitrogen tank and provides a stream of filtered nitrogen over a large area
Product such as: Entegris Wafergard Nitrogen Gun (Part# WGGB 01K AN)
Do not use a glass pipette for drying because the stream is too narrow
If nitrogen gun is not available, then cut a blue pipette tip so that the hole is approximately 4 mm wide and use this to direct nitrogen gas.
8. Filter sterilized purified water (npH2O)
Produced from water filtration device such as those obtained from Millipore
9. Microscope with 10x, 40x phase objectives to examine collagen fibril matrices created.
∗This protocol will work on any sufficiently smooth hydrophobic surface (water contact angle ≥ 78 °) (1).
†Disclaimer: Certain commercial products are identified in this protocol to adequately specify the experimental procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
Description
Preparation of matrices on a polystyrene surface
1.) Remove DPBS (1x), DPBS (10x), 0.1 M sodium hydroxide, collagen stock from 4 °C storage.
2.) Setup workspace in sterile hood with an appropriate workflow (example shown in Figure 1).
3.) Prepare Collagen Solution. Note: either mix this quickly, or prepare solution on Ice. Fibril formation in solution occurs rapidly if solution sits idle at room temperature or is prepared from warm solutions.
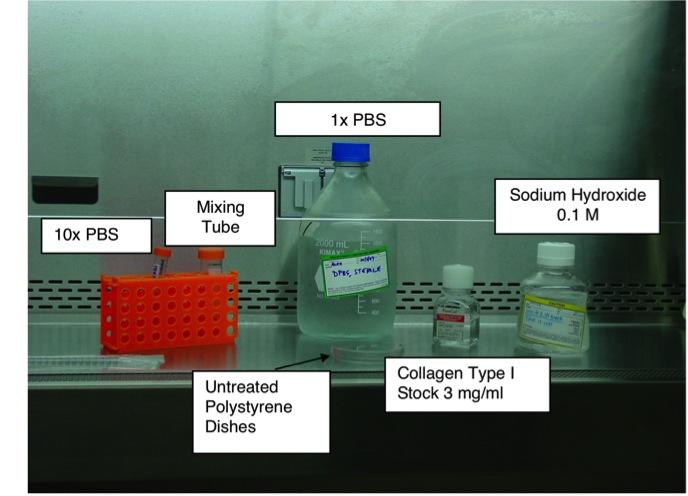
Figure 1. Schematic of general workflow for making collagen fibril matrices.
A general workflow for making a 24 mL total volume, neutralized collagen solution of 300 µg/mL collagen type I is as follows. The reader can scale these numbers to the total volume required for their experiment. We use the preparation at 300 µg/mL as we have determined that surface coverage on the dishes at this concentration provide cell responses similar to those seen in 3-D collagen gels (5).
1st add 21 mL of DPBS (1x) to mixing tube
2nd add 300 µL of DPBS (10x) to mixing tube
3rd add 2.4 mL Collagen Type I
4th mix solution gently by inverting several times
5th add 300 µL of 0.1 N NaOH
6th mix solution gently by inverting several times
7th add appropriate amount of solution to substrate (some suggested guidelines can be seen in Table 1).
Note: adding too little solution will result in an interconnected gel that may be difficult to remove from the culture dish without disrupting the thin matrix on the dish surface.
Table 1. Minimum suggested amounts of neutralized collagen type I solution to add to each well or dish. If making collagen matrices on different substrates placed within dishes or plates, e.g., 25 mm gold coated-hexadecane thiol treated cover slips placed in a 35 mm dish, then a general rule of thumb is that ~50% more solution should be added to the vessel.
| Type (Surface Area) | Minimum Amount of Solution (mL) |
| 35 mm dish, | 2.0 |
| 100 mm dish (58.1 cm²) | 12.0 |
| 12 well plate (3.8 cm²) | 1.5 |
| 24 well plate (2.0 cm²) | 1.0 |
| 48 well plate (0.75 cm²) | 0.8 |
| 96 well plate (0.32 cm²) | 0.3 |
4.) Allow dishes to incubate in a cell incubator at 37 °C for at least 16 hours.
5.) Examine solution and surface for presence of collagen fibril formation and compare to images shown in Figure 2.
6.) Remove dishes from the incubator and follow rinsing procedure below using sterilized Teflon squirt bottles to remove collagen gel from solution (diagram of hood setup in Figure 3).
Rinsing procedure:
1.) Sterilize squirt bottles prior to their first use, and at regularly scheduled intervals throughout the year as follows:
1st fill bottles with ethanol and spray down bottles with ethanol
2nd place bottles in sterile culture hood. Remove ethanol from bottles and allow to dry under sterile conditions. Wipe down external spray nozzle as well.
3rd rinse bottles at least 3x with sterile npH2O.
4th fill one bottle with DPBS (1x) and one bottle with npH2O.

Figure 2. Images of the collagen solution during the incubation process.

Figure 3. Schematic of general workflow for removing collagen gel from solution.
2.) Placing the wells at an angle in a tray (see Figure 4), rinse the surface with the DPBS squirt bottles, and then rinse with the npH2O squirt bottle. Aspirate the rinsing solution simultaneously using a sterilized pipette attached to a vacuum line. The object is to remove all of the bulk collagen gel that may remain on the surface. The npH2O rinse removes any salts from the surface.

Figure 4. Schematic of removing collagen gel from surface.
3.) Using the nitrogen gun with moderate pressure (~35 psi), and at a distance of about 10 cm from the sample, dry the matrix by blowing nitrogen over the surface. Randomize the drying process by moving the nitrogen source in a cross like fashion over the surface. If the stream of gas is directed it will cause the collagen fibrils to align on the surface (6). The drying process should take less than 40 seconds. One should be able to see a white haze on the surface of the substrate if the collagen fibril matrix has formed (Figure 5).
4.) Immediately place the matrix back into DPBS (1x) for storage at 4 °C. The matrices are good for at least one week.
5.) If one wishes to stiffen the collagen fibril matrices and produce altered cell responses, the matrices should have the DPBS (1x) storage solution removed, be rinsed with sterile npH2O, dried with a nitrogen gun in a fashion similar to step, then be left at the back of a running sterile culture for 24 hours (7, 8).

Figure 5. Example of white haze on the surface of the Petri dish after drying. Under a phase microscope, one should be able to observe the layer of collagen fibrils (Figure 6).

Figure 6. Phase images of collagen fibrils. A) 10x objective, Ph 1. B) 10x objective, Ph 1, taken near a portion of the surface that was masked so that no fibrils could grow. C) 40x objective, Ph 2. D) 40x objective, Ph2, taken near a portion of the surface that was masked so that no fibrils could grow.
References:
1. Elliott JT, Woodward JT, Umarji A, Mei Y, Tona A. The effect of surface chemistry on the formation of thin films of native fibrillar collagen. Biomaterials. 2007;28(4):576-85.
2. Keresztes Z, Rouxhet PG, Remacle C, Dupont-Gillain C. Supramolecular assemblies of adsorbed collagen affect the adhesion of endothelial cells. Journal of Biomedical Materials Research Part A. 2006;76A(2):223-33.
3. Hansen RR, Tipnis AA, White-Adams TC, Di Paola JA, Neeves KB. Characterization of Collagen Thin Films for von Willebrand Factor Binding and Platelet Adhesion. Langmuir. 2011;27(22):13648-58.
4. Liu Y, Goldberg AJ, Dennis JE, Gronowicz GA, Kuhn LT. One-Step Derivation of Mesenchymal Stem Cell (MSC)-Like Cells from Human Pluripotent Stem Cells on a Fibrillar Collagen Coating. PLoS ONE. 2012;7(3):e33225.
5. Elliott JT, Tona A, Woodward JT, Jones PL, Plant AL. Thin Films of Collagen Affect Smooth Muscle Cell Morphology. Langmuir. 2003;19(5):1506-14.
6. Amyot F, Small A, Boukari H, Sackett D, Elliott J, McDaniel D, et al. Thin films of oriented collagen fibrils for cell motility studies. J Biomed Mater Res B. 2008;86B(2):438-43.
7. McDaniel DP, Shaw GA, Elliott JT, Bhadriraju K, Meuse C, Chung K-H, et al. The Stiffness of Collagen Fibrils Influences Vascular Smooth Muscle Cell Phenotype. Biophys J. 2007;92(5):1759-69.
8. Chung K-H, Bhadriraju K, Spurlin TA, Cook RF, Plant AL. Nanomechanical Properties of Thin Films of Type I Collagen Fibrils. Langmuir. 2010;26(5):3629-36.
Collagen Fibril References From Our Group:
Elliott, J. T., A. Tona, J. T. Woodward, P. L. Jones, and A. L. Plant. 2003. Thin films of collagen affect smooth muscle cell morphology. Langmuir 19:1506-1514. [link]
Elliott, J. T., J. T. Woodward, K. J. Langenbach, A. Tona, P. L. Jones, and A. L. Plant. 2005. Vascular smooth muscle cell response on thin films of collagen. Matrix Biol. 24:489-502. [link]
Langenbach, K. J., J. T Elliott, A. Tona, D. McDaniel, and A. L. Plant. 2006. Thin films of Type I collagen for cell by cell analysis of morphology and tenascin-C promoter activity. BMC Biotechnol. 6. [link]
McDaniel, D. P., G. A. Shaw, J. T. Elliott, K. Bhadriraju, C. Meuse, K. H. Chung, and A. L. Plant. 2007. The stiffness of collagen fibrils influences vascular smooth muscle cell phenotype. Biophys. J. 92:1759-1769. [link]
Elliott, J. T., M. Halter, A. L. Plant, J. T. Woodward, and A. Tona. 2008. Evaluating the performance of fibrillar collagen films formed at polystyrene surfaces as cell culture substrates. Biointerphases 3:19-28. [link]
Amyot, F., A. Small, H. Boukari, D. Sackett, J. Elliott, D. McDaniel, A. Plant, and A. Gandjbakhche. 2008. Thin films of oriented collagen fibrils for cell motility studies. Journal of Biomedical Materials Research Part B-Applied Biomaterials 86B:438-443. [link]
Spurlin T.A., Bhadriraju, K., Chung, K., Tona, A. and Plant, A. 2009. The treatment of collagen fibrils by tissue transglutaminase to promote vascular smooth muscle cell contractile signaling. Biomaterials 30:5489-5496. [link]
Bhadriraju, K., Chung, K., Spurlin T.A., Haynes R.J. Elliott J.T., Plant, A.L. 2009. The relative roles of collagen adhesive receptor DDR2 activation and matrix stiffness on the downregulation of focal adhesion kinase in vascular smooth muscle cells. Biomaterials 30:6687-6694. [link]
Plant, A.L., Bhadriraju, K., Spurlin T.A., Elliott J.T. 2009. Cell response to matrix mechanics: Focus on collagen. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 5:893-902. [link]
Chung, K., Bhadriraju, K., Spurlin T.A., Cook, R.F., Plant, A.L. 2010. Nanomechanical properties of thin films of type I collagen fibrils. Langmuir 26:3629-3636. [link]
Anderton, C.R., DelRio, F.W., Bhadriraju, K., Plant, A.L. 2012. The effect of high vacuum on the mechanical and chemical properties of collagen fibril matrices. Langmuir in revision.
Christopher R. Anderton, Tighe A. Spurlin, and John T. Elliott wrote this protocol.
Contact john.elliott [at] nist.gov (john[dot]elliott[at]nist[dot]gov) for further questions.

