NIF - PEM Fuel Cells
Fuel cells are basically a refuelable battery. Recent breakthroughs in PEM fuel cells have turned the technology into a potential consumer product with applications as replacements for rechargeable batteries, to remote power for houses, to power for automobiles. Neutron imaging of PEM fuel cells allows one to analyze the mass flow of water through the GDL and out of the fuel cell through the flow channels. Water balance in a fuel cell is critical to the operation of the cell. Without proper humidification of the membranes the proton conduction will not work. Water that is formed at the cathode must leave the cathode quickly to allow oxygen to reach the cathode catalyst layer. This is where neutrons can play a role in analyzing the effectiveness of the gas diffusion layer and water channels in removing water from the fuel cell.
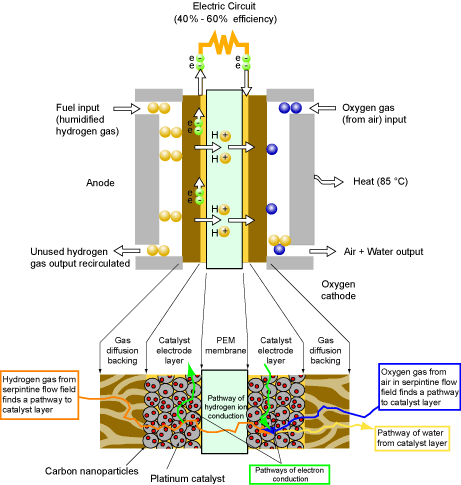
PEM Fuel Cell Basics
- Fuel cells are operationally equivalent to a battery.
- The reactants or fuel in a fuel cell can be replaced unlike a standard disposable or rechargeable battery.
- For automotive applications hydrogen is the fuel choice.
- Low temperature (~85 °C) Polymer Electrolyte Membrane (PEM) type cells are the standard devices.
- Electrochemical energy comes from the reaction: ½ H2 + ½ O2 → H2O.
- Theoretically the maximum voltage that this reaction can generate is 1.2 V. However, in practice the cell usually generates about 0.7 V to 0.9 V and about 1 W cm-2 of power.
Anode
- The fuel (hydrogen gas) is circulated through serpentine like channels or grooves machined in solid graphite.
- Hydrogen passes through a porous mesh layer called the gas diffusion media.
- Hydrogen makes contact with a thin layer of platinum catalyst embedded in graphite nanoparticles and electron is stripped from hydrogen.
- Electrons are conducted away through the graphite nanoparticle electrode layer to an electric circuit.
- Protons are conducted through a thin (20 µm to 100 µm) Polymer Electrolyte Membrane (PEM) (Nafion or Polysulfonic acid).
Cathode
- Same configuration as the anode except oxygen gas (from the air) is circulated through the gas flow region.
- Protons coming from the anode encounter oxygen and the electrons from the external circuit in a catalyst electrode layer (platinum embedded in carbon nanoparticles).
- Liquid water byproduct must now drain away so as to not block the fuel cell.
Contacts
-
(301) 975-6465
-
(301) 975-6207
-
(301) 975-4842

