Summary
Cell counting (or cell enumeration) is one of the most fundamental measurements in biotechnology, from biomanufacturing to medical diagnosis to advanced therapy. For example, many cell-based bioassays, including activity and potency, must be normalized to the cell number to allow data inter-comparability. The number of cells within a bioreactor may serve as a quality assurance metric in a manufacturing process. Cell number is critical for determining the proper dose of a cell-based therapy.
Cell counting also encompasses a wide range of measurement modalities applied to an even wider range of sample types for many different intended uses. This poses a challenge to developing a single standardized cell counting method or reference material to address all cell counting needs.
The NIST program aims to addresses the critical needs in cell counting including standardization and measurement assurance through our technical programs and coordination with stakeholders and standards development organizations (SDOs).
Description
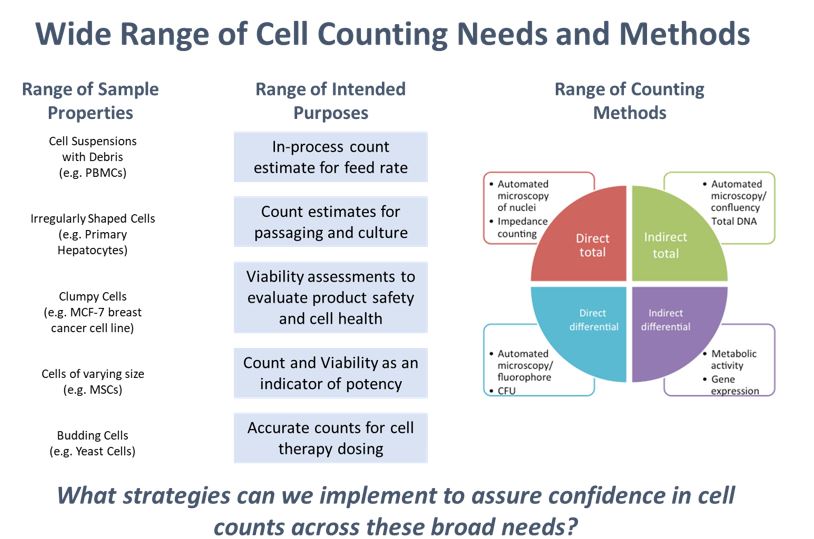
Recent Publications and Resources for Cell Counting and Related Measurements
- Tools
- NOW AVAILABLE: COMET (Counting Method Evaluation Tool), A Shiny Application that executes the statistical analysis and reporting outlined in ISO 20391-2:2019
- Standards
- ISO 20391-1:2018 Biotechnology — Cell counting — Part 1: General guidance on cell counting methods
- Now available at: https://www.iso.org/standard/68879.html
- ISO 20391-2:2019 Biotechnology — Cell counting — Part 2: Experimental design and statistical analysis to quantify counting method performance
- Now available at: https://www.iso.org/standard/67892.html
- ISO 23033:2021 Biotechnology — Analytical methods — General requirements and considerations for the testing and characterization of cellular therapeutic products
- Now available at: https://www.iso.org/standard/74367.html?browse=tc
- ISO 20391-1:2018 Biotechnology — Cell counting — Part 1: General guidance on cell counting methods
- Workshops
- Cell viability workshop at the Virtual Cell Therapy Analytical Development Summit (December 15th, 2020)
- Standards Coordinating Body (SCB) Realizing the Benefit of 21st Century Cures through Standards Development Workshop (March 18-19, 2019, Rockville, MD)
- NIST-FDA Flow Cytometry Workshop: Building Measurement Assurance in Flow Cytometry (October 25, 2017, Gaithersburg, MD)
- NIST-FDA Cell Counting Workshop: Sharing practices in cell counting measurements (April 10, 2017, Gaithersburg, MD)
- Publications
- Practical application of cell counting method performance evaluation and comparison derived from the ISO Cell Counting Standards Part 1 and 2. Y Huang, J Bell, D Kuksin, S Sarkar, LT Pierce, D Newton, J Qiu & LLY Chan. Cell & Gene Therapy Insights 2021; 7(9), 937–960 (DOI: 10.18609/cgti.2021.126)
- Establishing a reference focal plane using beads for trypan-blue-based viability measurements. Peskin A, Lund SP, Pierce L, Kurbanov F, Chan LL, Halter M, Elliott J, Sarkar S, Chalfoun J. J Microsc. 2021 Sep;283(3):243-258. doi: 10.1111/jmi.13037. Epub 2021 Jul 26. PMID: 34115371.
- Advancing measurement infrastructure for cell and gene therapy product development, Sumona Sarkar, Sheng Lin-Gibson, Current Opinion in Biomedical Engineering, 2021, Volume 20, 100329.
- Outcomes from a cell viability workshop: fit for purpose considerations for cell viability measurements for cellular therapeutic products, Pierce, L., Sarkar, S., Chan, L., Lin, B., Qiu, J., Cell and Gene Therapy Insights 2021; 7(4), 551-569
- Standards Landscape in Cell Counting: Implications for Cell & Gene Therapy (Cell and Gene Therapy Insights, 2019)
- Summary of the National Institute of Standards and Technology and US Food And Drug Administration cell counting workshop: Sharing practices in cell counting measurements (Cytotherapy, 2018)
- Evaluating the quality of a cell counting measurement process via a dilution series experimental design (Cytotherapy, 2017)
- Understanding and managing sources of variability in cell measurements (Cell and Gene Therapy Insights, 2016)
- Webinars
- Nexcelom Webinar on Demand: Applying ISO Cell Counting Standards for Cell and Gene Therapy Products
- ISCT Webinar: Viability Determination of Cellular Therapy Products - The Need for Controlled Objective Analysis (Organized by the ISCT North America Legal & Regulatory Affairs Committee, 2015)
Examples of NIST Technical Programs in Cell Counting
Technical Contacts: Sumona Sarkar, Ph.D. & Laura Pierce, M.S.
Evaluating the quality of cell counting measurements using a dilution series experimental design
Validating and evaluating a cell counting measurement process can be difficult because of the lack of appropriate reference material. In this project we developed an experimental design and statistical analysis approach to evaluate the quality of a cell counting measurement process in the absence of appropriate reference materials or reference methods. This approach served as the basis for the ISO 20391-2:2019 Biotechnology — Cell counting — Part 2 international standard. Recent work has focused on collaborations with a wide range of stakeholders to implement the approach for cell therapy and regenerative medicine applications. Visit the project page to learn more.
(Collaboration with Steven Lund, NIST Information Technology Laboratory (ITL))
Control Strategies for Image-Based Cell Counting/Viability Measurements
We are developing an approach to benchmark image quality in automated trypan-blue dye exclusion-based viability assays, to assure consistency in data analysis and reduce variability in cell viability results. This approach is based on the incorporation of a small number of beads into a cell counting sample, where the beads serve as a convenient tool for monitoring characteristics of the image acquisition process for quantitative optical microscopy. This approach is particularly useful in automated image-based count/viability measurements where cells are counted in a counting chamber which can be difficult to access by the user.
(Collaboration with Leo Chan, Nexcelom Inc. and Joe Chalfoun and Adele Penske, NIST Information Technology Laboratory (ITL))
- Relevant Publication: Establishing a reference focal plane using beads for trypan-blue-based viability measurements. Peskin A, Lund SP, Pierce L, Kurbanov F, Chan LL, Halter M, Elliott J, Sarkar S, Chalfoun J. J Microsc. 2021 Sep;283(3):243-258. doi: 10.1111/jmi.13037. Epub 2021 Jul 26. PMID: 34115371.
Strategies for Correlating Cell Health/Basic Function with Cell Viability Measurements
Cell viability is an important attribute for cell-based therapies. Many analytical methods are used to evaluate cell viability, yet growing evidence suggests that there can be very poor correlation between viability measurements and biological functions of cell products. This project will lead to increased understanding of the utility of various viability measurements and potentially contribute to standards development for cell-based regenerative medicine products. Our strategy is to correlate quantitative assays of cell health with quantitative viability measurements determined by several widely used methods. (Collaboration with Steven Bauer, Center for Biologics Evaluation and Research (CBER))
Design of Experiments (DOE) approaches to optimizing image-based cell counting/viability measurements
Design of experiments is a valuable tool in evaluating the sources of variability and robustness of measurements. We have applied this approach to evaluating the sensitivity of cell count and viability measurements to image analysis parameters in image-based cell counting methods. A fractional factorial orthogonal design was developed to evaluate 8 image analysis parameters across several cell conditions of varying cell health. DOE analysis was used to evaluate sensitivity to parameter settings and aid in optimization of parameter settings.
(Collaboration with James Filliben, NIST Information Technology Laboratory (ITL))

