1927: NBS gold leaf electroscope
Noah Dorsey gave a concise description of the electrometric methods used for relative measurements of radium in his book Physics of Radioactivity.
The simple electroscope consists of a metal case within which, and near its center, is supported in a vertical position a well-insulated metal strip to the top of which is attached a narrow strip of thin foil, preferably of gold leaf. This strip of foil is usually spoken of as the leaf. The strip of metal and the leaf constitute the insulated system of the electroscope. When the insulated system is electrically charged by a suitable switch passing through the wall of the case, the leaf is repelled by the strip, and is deflected from its normal, vertical position. In opposite sides of the case are windows through which the position of the leaf can be observed. Such observation is usually made by means of a microscope having in its eyepiece a ruled scale.
When intended for gamma ray measurements, the electroscope should be carefully screened on all sides except one with lead at least one inch thick, so that the air in the electroscope will be protected from scattered radiations that would otherwise enter it. For the same reason, the windows should be as small as is conveniently possible. The ionizing radiation of which the intensity is to be measured enter the electroscope through the unscreened side. In order to minimize the effect of the absorption of the radiation by the wall of the container and by the salt itself, the measurements should be based upon the hard, penetrating radiation emitted by radium-C. For this reason it is desirable that the radiations entering the electroscope be filtered through lead at least 15 mm thick so that very little of the soft gamma radiation from radium-B enters the electroscope.
When everything is ready, all radium preparations are placed at such distances and so screened that they produce as small an ionization in the electroscope as possible - the preparations to be compared must be so placed that they produce only a negligible ionization. The insulated system is then charged and insulated, and the time required for the image of the leaf to move over a few divisions near the middle of the scale in the microscope is determined. From this, the rate of drift of the leaf, in divisions per second, when there is no radium near the electroscope, is determined. This is called the natural, or the blank, drift. It results from imperfect insulation and slight residual ionization of the air.
Then the tube under test is placed in a suitable position, and the time required for the leaf to drift over a certain portion of the scale is determined. If the blank drift is subtracted from the rate of drift observed when the tube under test is in position, the difference will be the rate of drift due to the radiation from the tube; this is known as the corrected drift.
The tube under test is now removed to its former position where it does not affect the electroscope, and the standard tube is placed in exactly the same position previously occupied by the tube under test. Its corrected drift is determined in exactly the same way as was that for the tube under test.
The ratio of the two corrected drifts is equal to the ratio of the intensities of the two radiations; which, in turn, is equal to the ratio of the amounts of radium-C that are contained in the two tubes, provided that the absorption of the radiations by the walls of the two tubes is the same in both cases. Knowing the amount of radium-C in the standard, the amount in the tube under test can now be computed at once. Noah Dorsey

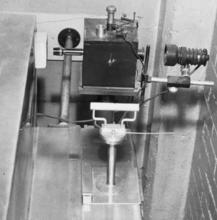
The photograph shown here from 1923 is an unidentified member of the radium section using the NBS electroscope. Dorsey did not include a schematic of the NBS gold-leaf electroscope in his textbook, but Leon Curtiss did publish an article on a projection electroscope about two years after arriving at the Bureau.

The NBS Standard Gold Leaf Electroscope. The ionization chamber consists of a 10 cm-cube free-air volume, with walls made of 1 cm thick lead sheet with ½ cm thick aluminum lining. A gold leaf is suspended near the center of the chamber and a quartz fiber, 10 µm in diameter, at the free end of the leaf provides a fine scale for optical projection, with a magnification of 100, onto a metric scale. Transit times of the quartz fiber image between two fixed points on the scale 6 cm apart are normally measured.

By the mid twenties the hazards of working with large quantities of radium were well understood by the experts, and Curtiss designed the radium calibration range shown here in such a way that the eyepiece could be observed through a telescope, which increased the distance between the observer and the high intensity sources under test.
Three decay schema from early publications by Rutherford and Soddy
In the decade that followed the pioneering work of the Curies to separate the new elements radium and polonium from pitchblende, a number of radiochemists and physicists in Europe and North America undertook intense investigations to understand the origins and nature of natural radioactivity. A description of these studies is beyond the scope of this article. By way of summary, however, we show three decay schema from early publications by Rutherford and Soddy. The most simplistic of these charts is from Rutherford's book of 1906. By 1911, Soddy and others had identified the main decay products in three distinct decay series corresponding to uranium, actinium and thorium. The last chart shows Rutherford's version of the uranium series in his 1936 book, The Newer Alchemy. With reference to these charts, we will now mention some of the chemical and physical properties of these decay products that are important in interpreting the response of the electroscope.
Mesothorium
The concept of isotopes, nuclides which have the same number of protons but differ in the number of neutrons, was understood quite early. This was essential for radium standards measurements because "mesothorium" was in fact another isotope, radium-228. Since radium-226 and radium-228 cannot be chemically separated, standards could only be prepared from ores that were rich in uranium but deficient in thorium. Both carnotite from Colorado and pitchblende from St. Joachimstal were satisfactory since they had a low thoria content.
Radon (Radium Emanation)

The decay chain for radium-226 is shown here. It was recognized quite early that one of the daughters in the decay chain of uranium was an unreactive gas. The half life of the "radium emanation" or radon-222 is 3.82 days. William Duane working with Marie Curie devised a radon "cow," whereby one could separate the gaseous radon-222 from the radium-226 parent. This generator could be "milked" every 20 days or so to yield a fresh sample of radon-222 which had nearly the same activity as the parent. This radon could be used in research, but it quickly was found to be useful in therapeutic applications. Glass tubes filled with radon gas soon became widely used in the United States and Europe in intracavitary brachytherapy.
The presence of radon in the radium decay series has implications for interpreting the response of the electroscope. First, as Dorsey noted, the electroscope responded primarily to gamma rays from Radium-C. Thus, one had to ensure that both the standard radium and the radium sample under test were in secular equilibrium with radon and its daughters, at least through Radium-C. In practice, this meant that new radium test samples had to be aged a minimum of 20 days in a sealed container prior to measurement with the electroscope.
Second, one can see that the electroscope could equally well be used to measure radon samples, because Radium-C is in equilibrium with radon within a matter of hours. In fact, NBS used the electroscope increasingly for radon measurements in the 20s and 30s.
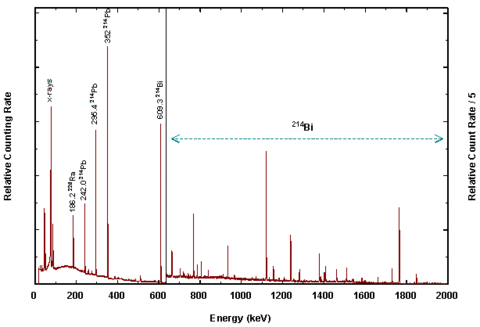
Radium-C (Bismuth-214)
Radium-C emits a gamma ray at 609 keV, which is the most abundant high energy gamma ray in the radium-226 decay series. The early investigators did not know the exact energy; they only knew that Radium-C emitted a very penetrating radiation. Dorsey listed half value thicknesses for Radium-C for a number of materials. The half value thickness for aluminum was given as 55 mm. The thick wall of the electroscope was designed to filter out softer (lower energy) gamma radiation from other nuclides in the radium series. These would be subject to more self attenuation in the sample itself.
All of these qualifications and restrictions on the use of the electrometric methods were well known to Marie Curie and Ernest Rutherford, and to Noah Dorsey and those who operated standards laboratories. As Dorsey noted in the quotation above, the method could only be used to measure the relative intensity of Radium-C gamma rays compared to Radium-C gamma rays from a standard of known mass. Another 30 years would pass before dosimetric standards were developed based on the quantity exposure measured in units of roentgen. Uncertainties in measurement included the mass and configuration of the radium salt, the thickness of the glass encapsulation, the positioning of the two sources (standard and test sample) relative to the electrometer, impurities in standard and test sample and the timing of the discharge of the electrometer. Dorsey describes in detail the importance of choosing two stopwatches and ensuring that they are clean and in good working order, and notes the uncertainty in timing with a stopwatch could be several fifths of a second.

