Summary

Gene therapy is rapidly growing field that utilizes viral and non-viral particles to deliver DNA or RNA to a patient’s target cells to treat intractable diseases, such as cancer and genetic diseases. The manufacturing of such particles is complex, potentially requiring months and resulting in heterogenous products with low yields of high-quality particles. Measurements of particle identity, purity, potency, safety, and stability are needed to ensure gene delivery system quality. NIST is developing approaches to provide advanced characterization of these critical quality attributes and of physical and infectious titer. NIST is also collaborating with federal and industry partners on developing and characterizing reference materials to make available to the public. These measurement tools and reference materials can help increase product understanding and accelerate gene delivery system development.
PAST EVENT
- NIST Workshops on Measurements and Standards for Advanced Therapy
- Day 1: NIST Gene Delivery Systems Public Workshop
November 1, 2023
8:30am - 5:00pm ET
- Day 1: NIST Gene Delivery Systems Public Workshop
Description

Viral Vectors
Viral vectors are a critical tool for delivering DNA or RNA into patient’s cells. Common viral vectors for gene therapy include adenovirus, lentivirus, and adeno associated virus. Administration of viral vectors can occur in vivo or ex vivo, such as for adeno associated virus or lentivirus CAR-T therapy, respectively. Heterogeneous production of viral vectors results in the presence of full, empty, or partially filled particles with therapeutic DNA or RNA. These varying states have immunogenic and functional impacts on a viral vector preparation. NIST is developing physical titer methods to quantitatively measure loaded viral particle state to provide manufacturers quality attributes on their viral vector preparation. Additionally, NIST is working on developing advanced measurements by imaging and flow cytometry of infectious titer to correlate with physical titer measurements. These physical and infectious titer measurements can provide holistic characterization of viral vector heterogeneity to advance understanding of viral vector quality.
FLOW VIROMETRY
Flow cytometry has traditionally been used to analyze cells, measure biomarkers or inner cellular molecules, or infected cells. Analyzing smaller entities, such as viruses, has shown to be extremely challenging; however, flow cytometry has become a powerful tool for their characterization because of the recent advancement in instrumentation and labeling reagent. The NIST Flow Cytometry Lab is utilizing the unique knowledge of staff and comprehensive collection of flow cytometers to conduct cutting edge measurements of viral vectors of ≥20 nm in size. Physical titer, viral packaging information, and potential contamination can all be quantitatively measured.

Viral and Non-Viral Particle Imaging

The goal of this project is to develop novel high-throughput light scattering microscopy to individually measure the mass of viral and non-viral particles to help elucidate particle loading, as well as size and concentration measurements, to create a robust and quantitative set of quality attributes for physical titer measurements.
See here for more project information.
VIRAL INFECTIVITY ASSAYS
Infectious titer is a critical measurement of viral vector function and potency. Traditional methods for infectious titer quantification include tissue culture infectious dose (TCID50) assays, plaque assays, and gene transfer assays as measured PCR and flow cytometry. NIST is developing protocols and improved measurement techniques which can reduce the variability and improve the reproducibility of infectious titer assays. Projects include automated plaque identification, lentivirus transduction, and pseudovirus infection and serum neutralization. Measurement methods include endpoint microscopy, time lapse imaging, and flow cytometry. High speed microscopy methods are under development to accelerate infectivity assay imaging.
See here for more information on pseudovirus and serology testing.

GENOMIC ASSAYS
Fast and robust viral vector characterization methods are significantly needed for the quality assurance of viral vector production. Traditional viral titer methods are often based on certain assumptions, such as 2000 molecules of p24 in each lentiviral particle. NIST is developing protocols and improved measurement techniques for viral vector genomic assays. These assays can reduce the sample handling time and variability and improve the reproducibility of viral vector titration.
NIST is also developing techniques and protocols to evaluate the viral vector genome integrity. The genome integrity was assessed by RT-ddPCR (such as LV) or ddPCR (such as AAV) assays targeted to distant regions of the LV genome as shown.

Other genomic assays for characterizing viral vectors:
- Testing for replication competent lentiviruses: ddPCR assay targeting VSVG.
- Important aspects of transducing activity are integration capacity, transgene expression and functionality by ddPCR assays targeting elements in the viral vector genome.
- Host nucleotide acids contamination: ddPCR assays targeting host reference gene (2 copies per genome), mitochondrial DNA (100 to 1000s copies per genome), or even Long interspersed nuclear elements (LINEs) (~1 million copies per genome).
Viral Vector Reference Materials
Reference materials can serve an important role is the development and commercialization of viral vector-based gene therapies. These materials can enable comparability of different viral vector preparations for physical and infectious titer measurements. NIST is collaborating with FDA and industry partners to characterize the next generation of viral vector reference materials. NIST is currently working on three reference material projects:
-

Illustration of adenovirus serotype 5 particle Adenovirus Serotype 5: NIST is collaborating with the BioProcessing Journal and the FDA to develop and characterize an adenovirus serotype 5 reference material. This material was originally produced in 2001. NIST performed final stability testing in 2021 (see here). NIST will coordinate interlaboratory testing for physical (as measured by OD260) and infectious titer (as measured by TCID50) to bridge measurements from the original and this new reference material. For more information or to participate in testing, please contact Jamie Almeida, jamie.almeida [at] nist.gov (jamie[dot]almeida[at]nist[dot]gov). See here for more information.
-

Illustration of lentivirus particle Lentivirus: NIST is collaborating with the BioProcessing Journal and the FDA to characterize a lentivirus reference material. This is a new reference material containing a GFP transgene. NIST will coordinate the safety and interlaboratory testing for physical titer (measured by a p24 ELISA assay) and infectious titer (measured by ddPCR or qPCR on transduced cells). For more information or to participate in testing, please contact Edward Kwee, edward.kwee [at] nist.gov (edward[dot]kwee[at]nist[dot]gov). See here for more information.
Vector Copy Number Reference Materials
The copy number of integrated transgenes into target cell genomes is critical for assessing the safety and efficacy of engineered cellular therapeutic products such as CAR-T cells. NIST is evaluating whether vector copy number cells (VCN) can be used to benchmark integrated lentivirus copy number measurements in target cells. These VCN cells also serve as a basis for developing cell-based (Jurkat) reference materials that can be distributed between laboratories to validate bioprocesses associated with the manufacturing and evaluation of cell and gene therapies.
- VCN Interlaboratory Study:. NIST is evaluating the suitability and utility of genomic DNA from lentiviral vector copy number (VCN) cells to serve as reference materials for measurement of integrated copy number into the genome of target cells. Assessment of interlaboratory data is currently underway. See here or contact Hua-Jun He (hua-jun.he [at] nist.gov (hua-jun[dot]he[at]nist[dot]gov)) and Zhiyong He (zhiyong.he [at] nist.gov (zhiyong[dot]he[at]nist[dot]gov)) for more information.
Fixed Cell Reference Material: Using VCN Jurkat cell lines as a foundation, NIST is establishing workflows to preserve cells with respect to fit-for-purpose measurements related to assay development, bioprocess validation, instrument benchmarking, and interlaboratory transfer of cell-based reference materials. Among fixatives to preserve the materials for single cell genomic measurements, NIST is also testing and characterizing various chemical cross-linkers to retain structural, morphological, and proteomic features at specific cell states (cell cycle, activation, DNA damage, viral infection) for flow cytometry and image-based analysis. Importantly, these fixed cells are stable and can be stored long-term or distributed between labs for comparison of data sets. For more information about fixed cell reference materials, please contact Mahir Mohiuddin (mahir.mohiuddin [at] nist.gov (mahir[dot]mohiuddin[at]nist[dot]gov)) or John Elliott (john.elliott [at] nist.gov (john[dot]elliott[at]nist[dot]gov)).
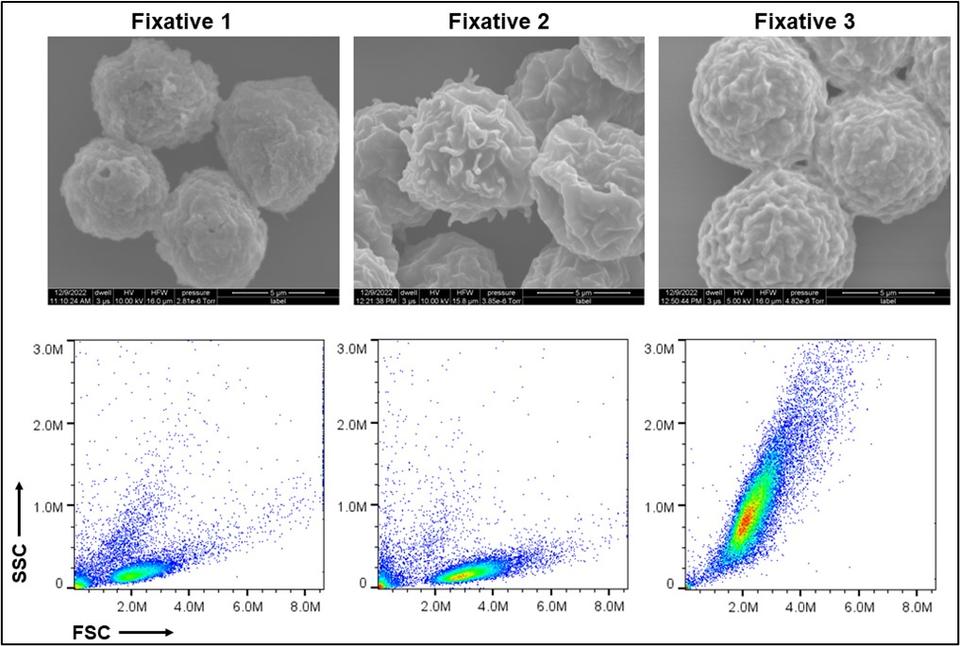
Structural and morphological properties of cells preserved with various fixatives. Scanning electron microscopy images (top) and scattering profiles from flow cytometry (bottom) are shown. Credit: Mahir Mohiuddin- Fixed Cell Reference Materials for Cell Viability: Cell viability is a critical attribute of cell samples with implications for both safety and effectiveness of cell-based products. A significant challenge in the standardization of cell viability assays is instrument-to-instrument comparability and interoperability of data sets. To help address these challenges, NIST is utilizing the fixed cell reference material and ERF (equivalent reference fluorophore) beads to establish transferable fluorescence scales that can be applied to image- and flow-based membrane integrity cell viability assays (e.g. AO/DAPI). The fixed cell reference material is fluorescently labeled and spiked into cell samples before executing existing cell count/viability protocols in order to establish benchmarked fluorescent scales for individual instruments. These benchmarks can be used to set and transfer gates around populations of live vs. dead cells between different instruments. By incorporating the use of standardized beads and these fixed cell reference materials, consistency in data analysis can be improved while variability between data sets is reduced. To learn more about this project, please contact Sumona Sarkar (sumona.sarkar [at] nist.gov (sumona[dot]sarkar[at]nist[dot]gov)) or Laura Pierce (laura.pierce [at] nist.gov (laura[dot]pierce[at]nist[dot]gov)).
- Genome-Based Cell Counting: Cell counting is fundamental measurement in the biosciences and is critical for cell-based assays, manufacturing control and, product release of cell-based products. There are many approaches to measure a cell count however there is no consensus on a reference method. Imaging and flow-based methods typically target the cell nucleus to distinguish and enumerate cells, however this relies on user-based gating of cells, debris, and various cell populations. In contrast, cell count based on the number of genome copies provides a biologically based definition of cells (genomic material inside of the nucleus) that is not subjective. Additionally, this approach can identify and count cell populations with specified genomic markers. We are implementing droplet digital polymerase chain reaction (ddPCR) to quantify the number of genome copies and then convert this value to a cell count. Digital PCR is becoming a more common technique in cell-based product manufacturing and testing, and this approach could allow the critical cell count value to be obtained simultaneously with other dPCR assays. For method development, we are using the VCN cells and targets for the HIV-1 Rev response element (RRE) in the integrated lentiviral provirus DNA, and human ribosomal protein L32 (RPL32) gene in the human genome. To learn more about this project, please contact Sumona Sarkar (sumona.sarkar [at] nist.gov (sumona[dot]sarkar[at]nist[dot]gov)).
- CAR-T VCN cell lines: NIST is working on the development of anti-CD19 CAR-T model cell lines by transducing Jurkat (Clone E6-1, ATCC) cells with a lentivirus expressing an anti-CD19 cassette and eGFP. The single cell clones of transduced Jurkat cell lines were selected, expanded, and validated by digital PCR to produce stable cell lines with N=1, N=2, N=3, and N=4 copies of the integrated provirus. The protein expression of anti-CD19 antibody and eGFP are being evaluated, and will be followed by functional study such as CAR-T killing assays. To learn more about this project, please contact Zhiyong He (zhiyong.he [at] nist.gov (zhiyong[dot]he[at]nist[dot]gov)), Linhua Tian (linhua.tian [at] nist.gov (linhua[dot]tian[at]nist[dot]gov)), Lili Wang (lili.wang [at] nist.gov (lili[dot]wang[at]nist[dot]gov)), or Hua-Jun He (hua-jun.he [at] nist.gov (hua-jun[dot]he[at]nist[dot]gov)).
Non-Viral Vectors
Extracellular vesicles (EVs) are nanometer-sized vesicles that contain bioactive lipids, RNAs and proteins, which can be transferred to recipient cells. EVs are important for physiological as well as pathological processes, such as coagulation and immune homeostasis, aiding cancer metastasis and spread of infectious diseases. These unique properties have spurred tremendous interest in the broad application of EVs in healthcare as potential disease biomarkers, regenerative medicines and therapies, and as drug delivery vehicles. Various characterization methods based on different principles have been developed for EV investigations, but their relatively small sizes and complex population and subpopulation heterogeneity, and lack of reference and/or control materials for purification and characterization pose key challenges to the successful translation of EVs to the clinic and beyond. The robust characterization methods identified in the EV work are applicable to other non-viral vectors including lipid nanoparticles (LNPs).
Research has been focused in four areas to develop some measurement assurance around EV technology:
- Develop standardized cell-based platforms to produce EV-based therapeutics.
- Develop EV reference materials that allow researchers/manufacturers to validate EV measurements and maintain measurement assurance throughout EV production and characterization.
- Develop standardized measurement systems for determining the molecular composition and biological activity of EVs by flow cytometry.
Emerging Project:
- Develop and apply robust EV isolation and analytical characterization measurements and tools to the analysis of human blood derived EVs.

Postdoctoral Opportunities
NIST is looking for postdoctoral applicants interested in developing and applying novel characterization approaches to measure gene delivery system quality. Two-year fellowships are available through the NIST-NRC Postdoctoral Fellowship program. The program is open to US Citizens only, offers competitive salary and benefits, with research proposal and application deadlines annually on February 1 and August 1. Contact NIST staff and see the NIST-NRC webpage for more information.
Standards Efforts
Documentary standards are needed to harmonize research, development, and commercialization of gene delivery systems. Under ISO TC 276, new standards are under development to standardize terminology and quantification methods for viral vectors and lipid nanoparticles. See here for information on how to join the US Technical Advisory Group to ISO TC 276 and contribute to these standards efforts.

