Summary
BECOME A MEMBER:
• Complete the Letter of Interest Form
• Participants will sign a Cooperative Research and Development Agreement (CRADA); government agencies may join under a Letter Agreement (LA)
• Annual fee of $25,000 or in-kind contribution of equivalent value
Notice of NIST's Flow Cytometry Standards Consortium in the Federal Register
Members of NIST Flow Cytometry Standards Consortium
UPCOMING EVENTS:
NIST–FDA–NIAID AI and Flow Cytometry Workshop, June 9 to 10, 2025
Description

Advances in cell and gene-based therapeutics as well as other regenerative medicine products have increased the need for high quality, robust, and validated measurements for cell characterization. Flow cytometry, including imaging cytometry, has emerged as an important platform due to its ability to rapidly and simultaneously characterize heterogeneous cell populations and subcellular analytes. For example, flow cytometry has been critical for establishing identity, purity, and potency for Chimeric Antigen Receptor (CAR)-T cell manufacturing; and associated data to support the approval of Biological License Applications (BLA) by the U.S. Food and Drug Administration (FDA) and the approval by the European Medicines Agency (EMA). In addition, multiparameter flow cytometric measurements are routinely carried out in vaccine, drug and cancer research, clinical diagnosis, and immunotherapies. However, challenges remain with respect to measurement confidence and comparability of measurement results from different instrument platforms, locations, and over time, hindering critical decision-making based on flow cytometry data in research and clinical settings. The NIST Flow Cytometry Standards Consortium aims to bring together experts across the regenerative medicine field including stakeholders in industry, academia, and government to address this need.
CONSORTIUM GOALS
- Develop reference standards including reference materials, reference data, reference methods, and measurement service for assigning the Equivalent Number of Reference Fluorophores (ERF) to calibration microspheres and assessing the associated uncertainties and utilities
- Develop candidate reference standards including biological reference materials, reference data, reference methods
- Design interlaboratory studies based on candidate reference materials to support the development of best practices and standard methods
Visit these pages to learn about Consortium
Membership, Events, and Impacts.
Consortium Working Group Activities
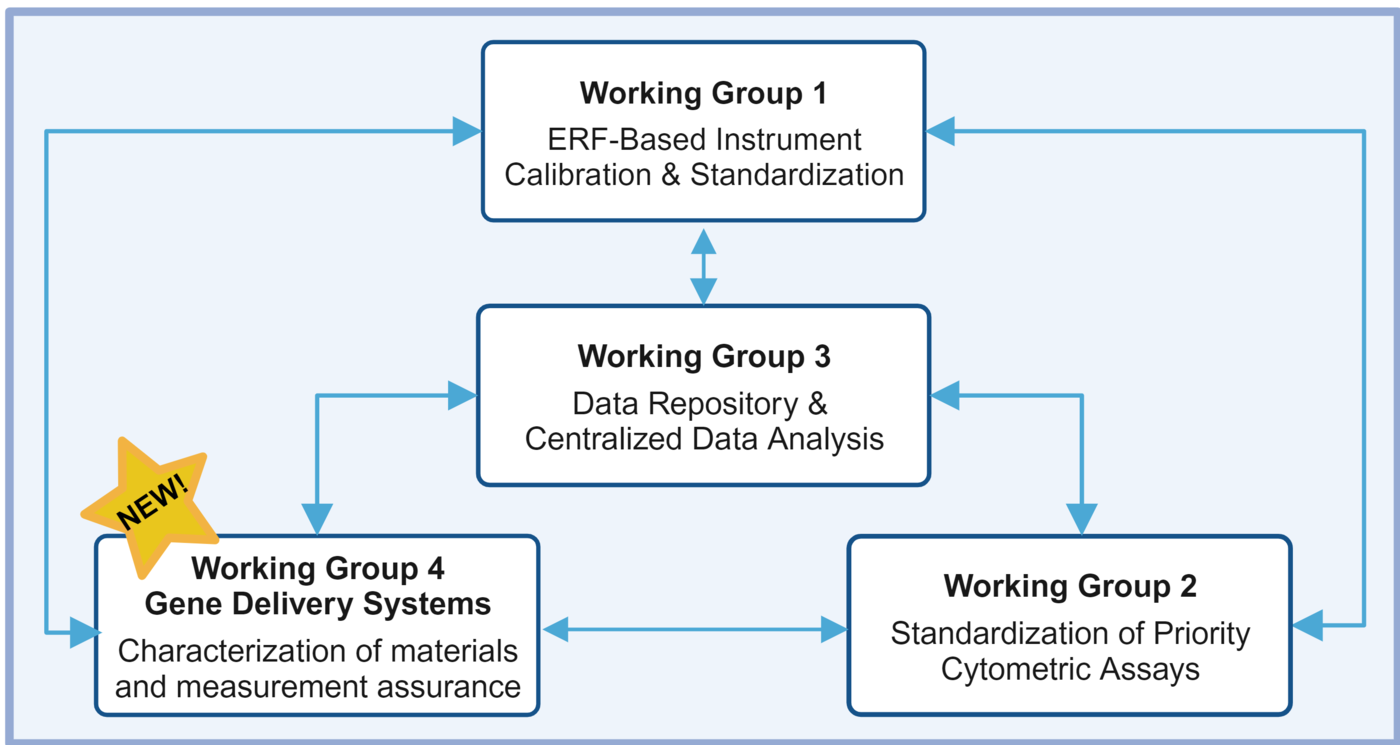
Four working groups (WG) have been formed under the consortium to drive the development of standards, measurement assurance tools, best practices and methods, and advanced capabilities.
- FCSC Working Group 1 – ERF-based Instrument Calibration and Standardization
- FCSC Working Group 2 – Flow Cytometry Assay Standardization
- FCSC Working Group 3 – Data Repository and Centralized Data Analysis
- FCSC Working Group 4 – Gene Delivery Systems
- FCSC Working Group 5 – Artificial Intelligence and Machine Learning Approaches (Launching Soon)

