Summary
Cells are being used in drug discovery, therapeutics development, biomedical research, and biotechnological and medical applications. However, the measurement challenges for these applications are complex and will be impeded by the complexity and dynamic nature of cells. The emergence of cell-based therapeutics has increased the need for high quality, robust, and validated measurements for cell product characterization. NIST serves as a neutral ground for the discussion of underpinning measurements and other manufacturing needs. Our effort aims to improve the confidence in cell measurements needed for translation and commercialization of cell-based products including cell therapy products (CTPs).
Description
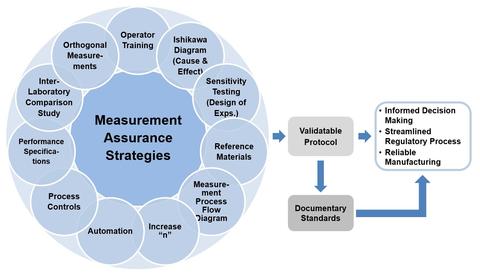
There are many strategies that may be employed to improve confidence in biological measurements. Measurement assurance can be achieved by taking steps to reduce uncertainty and improve confidence in the measurement result.
NIST collaborates with key stakeholders in industry, other government agencies, and academia to develop measurement assurance strategies and advanced measurement capabilities for cell characterization. Data and concepts developed through workshops and laboratory programs inform the development of standards.
Measurement Assurance concepts
Workshops and Meeting Materials
- NIST Workshop Report: Measurement Challenges for CAR-T Biomanufacturing
- NIST Workshop: Strategies to Achieve Measurement Assurance for Cell Therapy Products
- NIST-FDA Workshop: Cell Counting (2017)
- NIST-FDA Workshop: Flow Cytometry (2017)
- NIST-FDA Workshop: Genome editing (April 23-24, 2018)
- NIST-FDA Workshop: Cell Characterization (2018)
Cell Manufacturing Concepts
- Manufacturing Cell Therapies: The Paradigm Shift in Health Care of This Century
- Points to Consider for Cell Manufacturing Equipment and Components
Cell Measurements
Current Activities & Products
Prototype Cell Assay Measurement Platform (P-CAMP): This unique platform technology enables automated multimodal analysis of large parameter spaces and was designed to guide the development of measurement assurance strategies for assays used for characterization and testing of biological products and processes. The system design is centered on automation that can set up thousands of experiments for the purpose of identifying sources of variability in bioassays, designing fit-for-purpose reference materials, and generating specifications that assure confidence in an assay measurement system.
Cell Counting: Cell count is one of the most fundamental metrics of a cell sample. This measurement underpins key decisions in the manufacturing and commercialization of cell based products. While cell counting was once considered a routine and well-established measurement, the emergence of CTPs has required a greater understanding of and appropriate means to specify cell counting measurement quality. NIST has been working to develop strategies to improve the confidence of cell measurements critical for the translation, manufacturing and commercialization of CTPs.
Cell Line Authentication - The scientific community has responded to the misidentification of human cell lines with validated methods to authenticate cells. Currently, there is a consensus standard in place for human cell line authentication using short tandem repeat (STR) profiling which describes in detail the specific procedures to obtain reliable genotyping results. Databases of human STR profiles and commercial kits for human STR genotyping are also available. For non-human cell line authentication, however, there are no standards, STR genotyping kits, or databases available. To help address this need, NIST has established the Mouse Cell Line Authentication Consortium and is working with consortium members to test and validate NIST’s patented authentication method.
Fluorescence Microscope Benchmark - Fluorescence Microscopy is routinely used in laboratories to visualize cellular behavior and intracellular mechanisms. To use fluorescence microscopy as a quantitative tool (i.e. relative concentration), benchmarking materials and methods are required to ensure imaging is occurring under quantitative conditions. A fluorescence microscope benchmarking strategy has been deployed on the NIST webpages. The site includes step-by-step installation and use of open-source microscopy control software, examples of fluorescence reference materials and charting software to monitor several parameters describing microscope performance (sensitivity, linear range, field homogeneity, etc.). Access to the technical publication is also available at the site.
Assay Design Cause and Effect - An example of introducing quality metrics into a cell viability assay measurement was published in several articles described below. The quality was built around a nanoparticle cytotoxicity measurement due to the large variability in assay results described in the literature. The result of a cause-and-effect analysis of the MTS assay was an assay plate design that includes several control measurements to ensure the assay process performed as expected. The control measurements were used to develop specification that must be met to provide evidence for confidence in the nanoparticle cytotoxicity test result. The approach outlined in the cause and effect and the interlaboratory comparison manuscripts can be applied to cell based assays to improve confidence in the measurement result.
Use of Cause-and-Effect Analysis to Design a High-Quality Nanocytotoxicology Assay
Toward Achieving Harmonization in a Nano-cytotoxicity Assay Measurement through an Interlaboratory Comparison Study (Supplemental Protocol S1 – MTS cell viability assay with A549 (serum free treatment)
Toward Achieving Harmonization in a Nanocytotoxicity Assay Measurement Through an Interlaboratory Comparison Study, Supplementary Data

