MATES
Summary
The Multi-Agency Tissue Engineering Sciences (MATES) is an ad hoc interagency working group that provides a platform where federal agencies can interact efficiently and effectively to exchange information on tissue engineering and regenerative medicine. MATES members coordinate efforts related to cell therapy, gene therapy, biomaterials, tissue engineering, regenerative medicine and organ on a chip from research and development to commercialization. MATES holds monthly meetings to exchange information.
GOALS
- Facilitate communication across departments/agencies
- Enhance cooperation and collaboration among federal agencies
- Identify gaps and needs for tissue engineering and regenerative medicine research
- Facilitate communication with regard to translation of research advances into practical applications
- Monitor advances and identify and communicate needs for new technologies, standards and manufacturing science.
HISTORY
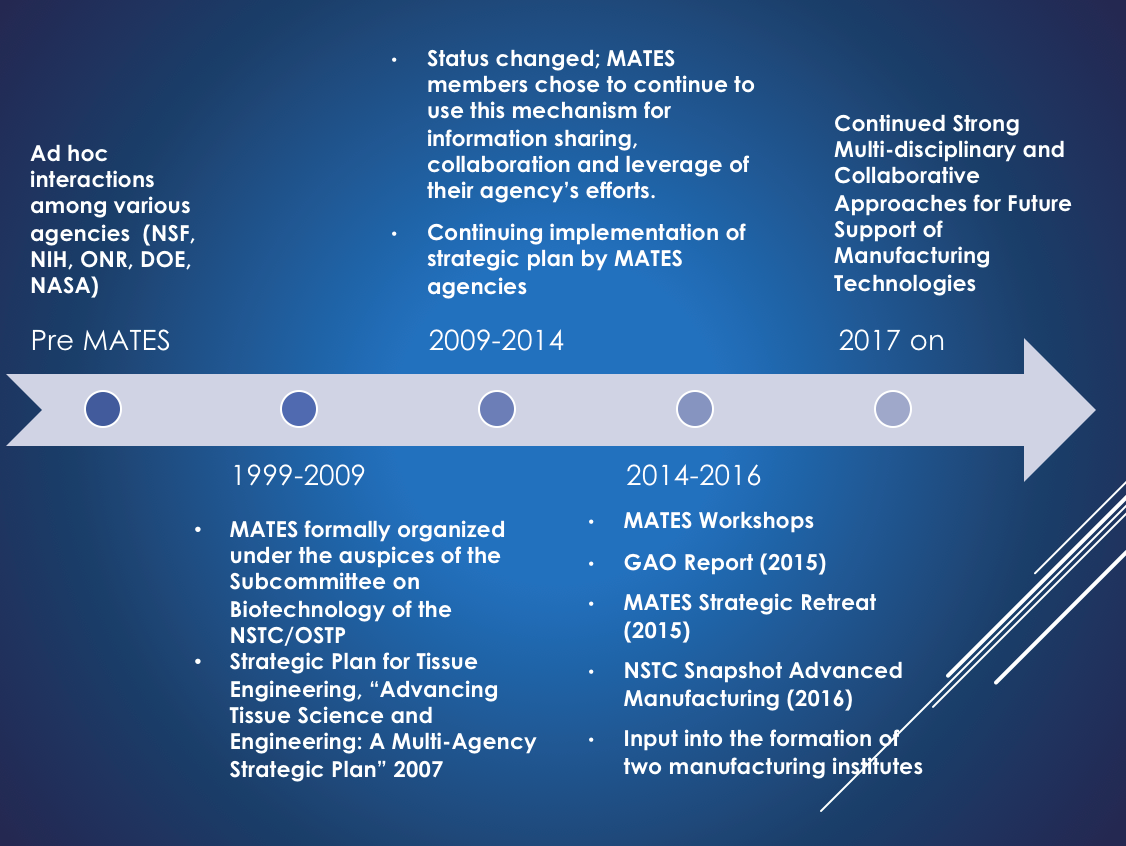
The MATES Interagency Working Group (IWG) was organized under the auspices of the Subcommittee on Biotechnology of the National Science and Technology Council (NSTC) in 2000 to serve as the means by which federal agencies involved in tissue engineering could stay informed of each other’s activities and coordinate their efforts in a timely and efficient manner. Since 2007, MATES has operated as an ad hoc interagency working group that continues with its original mission.
CURRENT PARTICIPATING AGENCIES
- National Institutes of Health (NIH)
- Food and Drug Administration (FDA)
- National Science Foundation (NSF)
- Veterans Affairs (VA)
- National Institute of Standards & Technology (NIST)
- Department of Defense (DoD)
- National Aeronautics and Space Administration (NASA)
MATES OUTPUT & ACTIVITIES
- Hold monthly meetings since 2000
- Archived 2008 MATES Website
- Advancing Tissue Science & Engineering: A Multi-Agency Strategic Plan (2007)
- MATES played a key role in providing the data for the following report:
- GAO-15-553: United States Government Accountability Office, Report to Congressional Requesters, Regenerative Medicine: Federal Investment, Information Sharing, and Challenges in an Evolving Field, 2015.
- Workshops and Symposia Organized since 2012
- 2012 FDA-NIST-NIH Workshop, Gaithersburg, MD: Functional Imaging for Regenerative Medicine
- 2014 TERMIS Workshop, Washington, DC: Government Efforts on the Path to Patients for Regenerative Medicine Therapies: A MATES Symposium
- 2015 TERMIS Workshop, Boston, MA
- 2016 TERMIS Symposia, San Diego, CA: The TERM Ark: A shipMATES Perspective on Emerging Trends in Government Support
- 2017 TERMIS Symposium, Charlotte, NC: Cell and Tissue Manufacturing Innovation
- Hunsberger J, Lundberg M, Allickson J, Simon Jr CG, Zylberberg C, Beachy S (2019) Examining resources, initiatives, and regulatory pathways to advance regenerative medicine manufacturing. Current Stem Cell Reports 5, 162-172. DOI: org/10.1007/s40778-019-00163-0
- MATES coordination played an integral role in the creation of two Manufacturing USA Institutes:
- National Institute for Innovation in Manufacturing Biopharmaceuticals (NIIMBL)
- Advanced Regenerative Manufacturing Institute (ARMI|BioFabUSA)
MATES CHAIRS
2000 - 2004: Fred Heineken (NSF)
2004 - 2014: Christine Kelley (NIH)
2014 - 2017: Richard McFarland (FDA)
2017 - 2020: Martha Lundberg (NIH), Sheng Lin-Gibson (NIST)
2020 - 2024: Steven Becker (NIH), Carl Simon (NIST)
2024 - present: Luisa Russell (NIH), Laura Ricles (FDA)
Disclaimer: NIST is a member of MATES and hosts the MATES website as a part of its contribution to this ad hoc interagency working group. The presence of content on this MATES website does not constitute an endorsement by any of the participants or the participating federal agencies.
Contacts
Chair
-
(301) 975-8574

