Biomaterial Measurements

Strips wells containing hydrogel scaffolds seeded with human cells.
A biomaterial* is a material designed to take a form that can direct, through interactions with living systems, the course of any therapeutic or diagnostic procedure. We develop standards, methods, tools and technology to advance the reliability of biomaterials for use in the biomedical industry, tissue engineered medical products and advanced therapies.
Current Activities & Products

| Measuring Cell Viability in Scaffolds: We are developing a model scaffold-cell-assay system to serve as a guide for validating measurements of cell viability in a scaffold. |

| Real-time Measurement of Multiple Polymerization Properties: NIST Standard Reference Instrument (SRI 6005) is a tensometer for measuring polymerization kinetics of biomedical polymers. |

| SRM 2910b Calcium Hydroxyapatite Reference Material: This Standard Reference Material (SRM) is intended for use in evaluating the physical and chemical properties of apatites, which are often used as a biomaterial. |

| Protocol for Preparing Fibrillar Collagen Matrices with Robust and Reproducible Cell Responses: A detailed and highly-vetted protocol for preparing fibrillar collagen substrates will well-defined properties for cell culture. |

| DiameterJ Plugin for Measuring Fiber Diameter in Tissue Engineering Scaffolds: A validated, open-source tool for automated analysis of scanning electron micrographs for determining fiber diameter. |
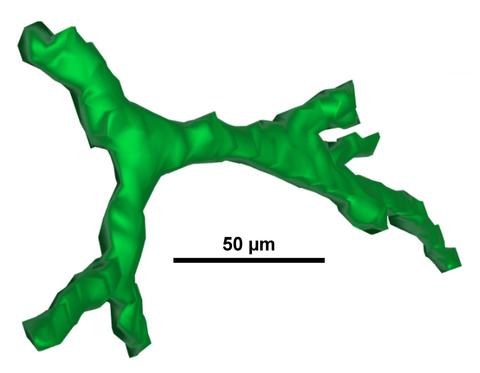
| 3D Cell-Scaffold Interactions: New methods for assessing how 3D scaffold structure influences 3D cell shape are being developed. |

| Combinatorial Cassettes for Higher-Throughput Screening of Osteogenesis in Mice: Combi-cassettes are planar structures with many holes that can be loaded with various cell-scaffold constructs and implanted into animals for the identification of formulations that induce tissue regeneration. |

| ASTM Committee F04: ASTM F04 has and is developing many standards for biomaterials that are used in tissue engineering and regenerative medicine. |
* Zhang X, Williams D (2019) Definitions of Biomaterials for the Twenty-First Century. Elsevier.
Created February 14, 2020, Updated April 10, 2025

